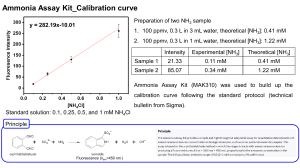
Part II. Short Answer. Please show all work and report all numerical
advertisement
Part II. Short Answer. Please show all work and report all numerical answers to the correct number of significant digits. Make sure all numerical answers have units written after them. 1. Compare and contrast the molecules NH3 and BH3 in terms of Lewis structures, three dimensional shape, polarity of bonds and polarity of the molecules. Include diagrams of each in your answer, including the Lewis structure and the three dimensional shape diagrams, including drawing in the bond dipoles above the bonds. In addition, state the predominant type of intermolecular attractive force that would exist between the molecules in each compound 2. In the space to the left of each substance, identify the substance as either ionic or molecular covalent. Then, write either the name or the formula for the compound I or M Chemical Formula P205 Chemical Name Calcium phosphate CuNO3 Potassium dihydrogen phophate B2H6 Tin (IV) carbonate 3. Balance the following two equations: ____ Na2S2O3 + ____ I2 ____ NaI + ____ Na2S4O6 ____ LaCl3 + ____ K2CO3 ____ KCl + ____ La2(CO3)3 4. What is the mass in grams of 1.20 X 1024 atoms of He? 5. Use the following unbalanced equation to answer questions a-c. _____N2 + _____H2 _____NH3 a. How many moles of nitrogen are needed to react with 24 moles of Hydrogen? b. Find the number of moles of ammonia that are produced from 500.0 g of nitrogen. c. Calculate the mass of hydrogen needed to produce 500.0 g of ammonia. 6. Ammonia, NH3, can be also be synthesized by the following reaction: 2 NO + 5 H2 2 NH3 + 2 H2O a) If 46.0 grams of NO and 12.5 g of H2 are reacted together, which is the limiting reactant b) Using your answer from part (a), what is the theoretical yield of ammonia, NH3, for this reaction? c) If only 22.6 g of ammonia was recovered, what is the % yield of this reaction?