chapter 12 notes
advertisement

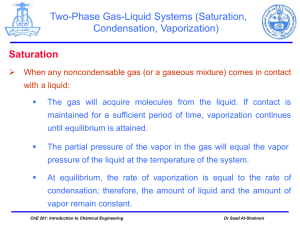
CHAPTER 12: LIQUIDS AND SOLIDS I. Properties of Liquids particles are close together and lower KE than gases intermolecular attractive forces are significant (definite volume) can flow (shape of container) high density compared to gases relatively incompressible will diffuse into another liquid if it can dissolve in it Surface tension force that tends to pull parts of a liquid’s surface together (attraction toward the center of the liquid) cohesion Adhesion liquids can be attracted to the sides of their containers can move up narrow tubes (until gravity stops it) capillary action Phase changes: vaporization: liquid gas (heat must be ABSORBED) o evaporation: occurs at surface of liquid (not boiling) o boiling: occurs throughout the liquid o COOLING PROCESS freezing: liquid solid (heat must be REMOVED) II. Properties of Solids particles very closely packed intermolecular attractive forces are VERY significant (definite volume) particles in fixed positions (vibrational motion possible) high density incompressible very low rate of diffusion Two Types: crystalline solids (crystals) o particles arranged in orderly, repeating, geometric pattern (page 369 Fig. 12-8) o definite, rigid shape o definite melting point (depends on type of atoms involved—see page 370 Table 12-1) amorphous solids (supercooled liquids) o no distinct geometric shape o can change shape (particles arranged randomly) III. Phase Diagrams For a pure substance IN A CLOSED CONTAINER, a phase diagram will show the conditions under which the phases of a substance exist (are stable). graph of pressure (y-axis) vs. temperature (x-axis) triple point: all 3 phases exist and are stable critical temperature (tc): highest temperature that the liquid phase can exist at critical pressure (Pc): LOWEST pressure that the liquid can have when at the critical temperature critical point: point where the critical temperature and critical pressure intersect NOTE: Denser phase (solid or liquid) will have greater area IV. Phase Changes When a substance changes phases, several things MUST happen: the temperature of the substance stays CONSTANT there must be a change in ENERGY complementary phase changes occur at the same T Heating Process (energy released to ENVIRONMENT) freezing (liquid solid + heat) condensation (gas liquid + heat) deposition (gas solid + heat) Cooling Process (energy absorbed from ENVIRONMENT) melting (solid + heat liquid) vaporization (liquid + heat gas) sublimation (solid + heat gas) V. Equilibrium Systems In a closed system (matter cannot enter or leave), a dynamic condition can arise: forward reaction reverse reaction Example: water in a closed container will evaporate, but the water level will not change very much because condensation also occurs The water level is eventually becomes constant, even though BOTH processes are taking place because: forward rate = reverse rate When this condition arises, a state of EQUILIBRIUM is reached. Le Chatelier’s Principle If a system at equilibrium is subjected to a stress, the system will SHIFT to try and relieve that stress (get rid of it). Example: liquid + heat vapor Stresses: Increase T of system: Decrease T of system: Add vapor: Remove vapor: Decrease V (increase P) Increase V (decrease P) System Shifts: VI. Vapor Pressure As a liquid evaporates, molecules at the surface of the liquid escape into the gas phase with an upward force. This force causes an upward pressure. *** Vapor Pressure: pressure caused by the evaporation of a liquid. as T increases, the average KE of the molecules in the liquid increases more molecules can escape into the vapor phase vapor pressure increases Vapor pressure also depends on the strength of intermolecular forces in the liquid. weaker the forces, HIGHER the v.p. Volatile liquids—high vapor pressure (evaporate quickly) VII. Boiling A liquid will boil when V.P of the LIQUID = ATMOSPHERIC PRESSURE NOTE: the highest temperature a liquid can get is its boiling point. Boiling point: TEMPERATURE when v.p. = a.p. Normal b.p.: temperature when v.p. = 1 atm VIII. Heats of Fusion and Vaporization Molar heat of FUSION: the amount of heat energy needed to MELT one MOLE of a substance at its melting point. Molar heat of VAPORIZATION: the amount of heat energy needed to VAPORIZE one MOLE of a substance at its boiling point. NOTE: all energy added goes toward breaking intermolecular attractions (T does not change during a phase change) Units: kJ/mol Values will depend on the strength of the intermolecular forces (stronger the force, larger the value) Molar heat of Solidification: the amount of heat energy RELEASED when one MOLE of a substance FREEZES. Molar heat of Condensation: the amount of heat energy RELEASED when one MOLE of a substance CONDENSES. NOTE: heat of solidification = -heat of fusion heat of vaporization = -heat of condensation For water (at 1atm): molar heat of fusion = 6.009 kJ/mol molar heat of vaporization = 40.79 kJ/mol 1 mole H2O = 6.009 kJ 1 mole H2O = 40.79 kJ Problem: How much heat will be needed to melt 3.5 moles of water at its melting point? How much heat will be needed to boil 3.5 moles of water at its boiling point? IX. Water Structure: Properties: polar molecule strong hydrogen bonding between molecules MM = 18.01 g/mol liquid at room temperature relatively low vapor pressure relatively high b.p. high heat of fusion high heat of vaporization high surface tension solid less dense than liquid phase Density of water: Temperature 0oC (solid) 0 oC (liquid) 3.98 oC NOTE: Density of water will DECREASE above 3.98 oC. Structure of ICE: Density (g/cm3) 0.917 0.99984 1.000