Unit 5 Phase Changes power point 2014
advertisement
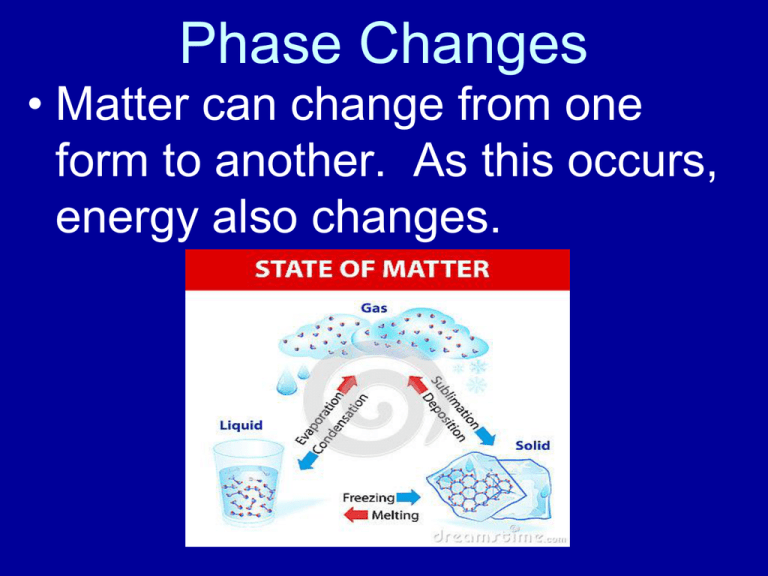
Phase Changes • Matter can change from one form to another. As this occurs, energy also changes. *As one proceeds from ice to water to water vapor, there is an increase in kinetic energy. * The changes of phase are not chemical, they are physical changes. Heating and Cooling Curves Heating Curve If there is a change in the temperature there is a change in kinetic energy because there is a change in the average motion of the particles. During a phase change there is no change in temperature, therefore no change in kinetic energy. Instead, energy goes to breaking bonds so it is a potential energy change. Energy Changes Along AB KE, no PE BC no KE, PE CD KE, no PE DE no KE, PE EF KE, no PE A cooling curve would be the opposite. Gases • Gases (g): Transparent, compressible, expand without limit, have no shape/volume. **Take the shape and volume of their container. Gases exert pressure: STP: defined as standard temperature and pressure *Found on Table A 101kPa or 1atm *Pressure can also be 760 torr or 760 mm Hg Liquids • Liquids: no definite shape/ but definite volume with very low compressibility. • Compressibility is the ability to occupy less space. Boiling Point • The temperature at which the vapor pressure of a liquid reaches atmospheric pressure; therefore allowing particles to escape as a gas. *When vapor pressure of a liquid = atmospheric pressure As atmospheric pressure increases one must raise the vapor pressure of the liquid by increasing its temperature. Normal Boiling Point is measured at standard pressure For water it is: 100C or 373K Vapor Pressure • • • See Table H The pressure exerted by the vapor evaporating off the surface of a liquid. Each liquid has its own vapor pressure. • As the temperature of the liquid increases, the vapor pressure of that liquid increases. Evaporation • The change of phase from liquid to gas. Heat of Vaporization: The amount of heat energy required to vaporize a given mass of a liquid to gas at a constant temperature. This is an endothermic process, energy is being absorbed. Each substance has its own Heat of Vaporization. For water at its normal boiling temperature of 100C and standard pressure the heat of vaporization is 2260 joules per gram. (Table B) Condensation • The change of phase from gas to liquid. This is an exothermic process. • The Heat of Condensation is the direct opposite of the heat of vaporization. The quantity of heat energy is the same as for the heat of vaporization, but instead of being absorbed the heat energy is being released. Solids • Definite shape/definite volume. ***Regular Geometric Pattern*** Melting (fusion): An endothermic process in which a solid becomes a liquid. For water at Standard Pressure the temperature at which melting occurs is 0C / 273K. Freezing (Solidification) • Freezing is the direct opposite of melting, but instead of being an endothermic process where energy is absorbed, it is an exothermic process where energy is released. Water freezes at 0C / 273K Heat of Fusion • The amount of heat energy required to change a given mass of solid to liquid at a constant temperature. Each substance has its own Heat of Fusion. For water at standard pressure, this quantity of heat is 334 J/gK (Table B) Heat of Solidification (Crystallization) • The direct opposite of the Heat of Fusion. Since solidification is an exothermic process, the heat energy is released instead of absorbed. Sublimation • The change of phase from solid to gas, completely skipping the liquid phase. • This generally occurs only in solids with high vapor pressures and weak intermolecular forces of attractions. • Examples: Dry ice (CO2), • paradichlorobenzene (moth balls), I2