HH#05.6-Lewis Structures
advertisement
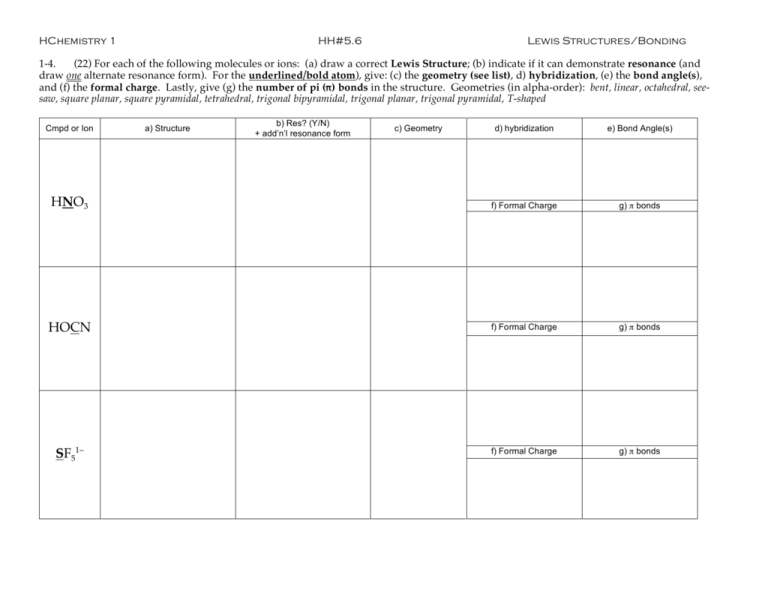
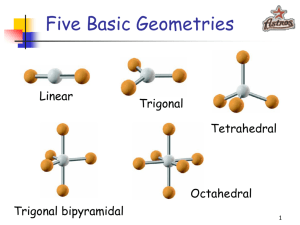
HChemistry 1 HH#5.6 Lewis Structures/Bonding 1-4. (22) For each of the following molecules or ions: (a) draw a correct Lewis Structure; (b) indicate if it can demonstrate resonance (and draw one alternate resonance form). For the underlined/bold atom), give: (c) the geometry (see list), d) hybridization, (e) the bond angle(s), and (f) the formal charge. Lastly, give (g) the number of pi (π) bonds in the structure. Geometries (in alpha-order): bent, linear, octahedral, seesaw, square planar, square pyramidal, tetrahedral, trigonal bipyramidal, trigonal planar, trigonal pyramidal, T-shaped Cmpd or Ion a) Structure b) Res? (Y/N) + add’n’l resonance form c) Geometry d) hybridization e) Bond Angle(s) HNO3 f) Formal Charge g) π bonds HOCN f) Formal Charge g) π bonds SF51– f) Formal Charge g) π bonds Cmpd or Ion CH2NH 5. (4) compounds. a) Structure b) Res? (Y/N) + add’n’l resonance form c) Geometry d) hybridization e) Bond Angle(s) f) Formal Charge g) π bonds What does “VSEPR” stand for? Briefly describe how VSEPR works and what this theory allows us to predict for molecular 6. (4) Consider the carbon-oxygen bond in formaldehyde (H2CO) and carbon monoxide (CO). In which molecule is the CO bond longer? In which is the CO bond stronger? Explain your responses.