Ch01-bond-ques-09a
advertisement

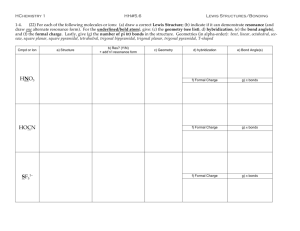
Chapter 1 Electronic Structure and Bonding Acids and Bases ~ 0.1 nm Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters • 1 nm = 10 Å • An atom vs. a nucleus ~10,000 x larger Nucleus = 1/10,000 of the atom Question 1 • What is the electronic configuration of carbon? • A) 1s2 2s2 2px2 • B) 1s2 2s2 2px1 2py12pz0 • C) 1s2 2s2 2px12py12pz1 • D) 1s2 1px1 1py12s2 Electron Configurations Noble Gases and The Rule of Eight • When two nonmetals react to form a covalent bond: They share electrons to achieve a Noble gas electron configuration. • When a nonmetal and a metal react to form an ionic compound: Valence electrons of the metal are lost and the nonmetal gains these electrons. G.N. Lewis Photo Bancroft Library, University of California/LBNL Image Library Footnote: G.N. Lewis, despite his insight and contributions to chemistry, was never awarded the Nobel prize. Notes from Lewis’s notebook and his “Lewis” structure. http://chemconnections.org/organic/Movies%20Org%20Flash/LewisDotStructures.swf Ionic Compounds • Ionic compounds are formed when electron(s) are transferred. • Electrons go from less electronegative element to the more electronegative forming ionic bonds. Covalent Compounds •Share electrons. •1 pair = 1 bond. •Octet rule (“duet” for hydrogen) •Lewis structures: Notice the charges: In one case they balance, can you name the compound? In the other they do not, can you name the polyatomic ion? More about “formal” charge to come. Question 2 • Select the correct Lewis structure for methyl fluoride (CH3F). • A) B) • • C) D) Important Bond Numbers (Neutral Atoms / Normal electron distribution) one bond two bonds three bonds four bonds H F Cl Br O N C I Question 3 • What is the correct Lewis structure of formaldehyde (H2CO)? • A) B) • C) D) Question 4 • Which of the following contains a triple bond? • A) SO2 • B) HCN • C) C2H4 • D) NH3 Formal Charge Formal charge is the charge of an atom in a Lewis structure which has a different than normal distribution of electrons. Important Bond Numbers (Neutral Atoms / Normal electron distribution) one bond two bonds three bonds four bonds H F Cl Br O N C I Important Bond Numbers (Neutral Atoms / Normal electron distribution) Organic Chemistry C H O N # of Valence e s 4 1 6 5 Total # of Bonds (neutral atom) 4 1 2 3 - Combinations of bonds (neutral atom): # of single bonds 4 2 1 1 2 0 3 1 0 # of double bonds 0 1 0 0 0 1 0 1 0 # of triple bonds 0 0 1 0 0 0 0 0 1 Total Bonds 4 4 4 1 2 2 3 3 3 # of Free Pairs of electrons 0 0 0 0 2 2 1 1 1 Formal Charge Formal charge = number of valence electrons – (number of lone pair electrons +1/2 number of bonding electrons) • • Equals the number of valence electrons (Group Number of the free atom) minus [the number of unshared valence electrons in the molecule + 1/2 the number of shared valence electrons in the molecule]. Moving/Adding/Subtracting atoms and electrons. HNO3 Nitric Acid Complete the following table. It summarizes the formal charge on a (“central”) atom for the most important species in organic chemistry. Question 5 • What is the formal charge of the carbon atom in the Lewis structure? • A) -1 • B) 0 • C) +1 C • D) +2 Question 6 • What is the formal charge of the oxygen atom in the Lewis structure? • A) -1 • B) 0 • C) +1 • D) +2 Resonance Resonance Resonance is a very important intellectual concept that was introduced by Linus Pauling in 1928 to explain experimental observations. Eg. SO2 Bond order 1.5 Bond length > double bond; < single bond TUTORIAL Resonance •Two or more Lewis structures may be legitimately written for certain compounds (or ions) that have double bonds and/or free pairs of non-bonded electrons •It is a mental exercise in “pushing” or moving electrons. •Refer to Table 1.6 Rules of Resonance Step 1: The atoms must stay in the same position. Atom connectivity is the same in all resonance structures. Only electrons move. NON-Example: The Lewis formulas below are not resonance forms. A hydrogen atom has changed position. H N H OH O N C H H C H Rules of Resonance • Step 2: Each contributing structure must have the same total number of electrons and the same net charge. • Example: All structures have 18 electrons and a net charge of 0. H O N H H C O N H H O H N C H H C H Rules of Resonance • Step 3: Calculate formal charges for each atom in each structure. • Example: None of the atoms possess a formal charge in this Lewis structure. H O N H C H Rules of Resonance • Step 4: Calculate formal charges for the second and third structures. • Example: These structures have formal charges. NOTE: They are less favorable Lewis structures. H O N H O H N C H H C H “Pushing” Electrons •same atomic positions H H C •differ in electron positions H .. .. + O O: H C O .. N .. .. H more stable Lewis structure N .. H less stable Lewis structure .. – O .. : “Pushing” Electrons •same atomic positions H H C •differ in electron positions only H .. .. + O O: H C O .. N .. .. H more stable Lewis structure N .. H less stable Lewis structure .. – O .. : Why use Resonance Structures? •Delocalization of electrons and charges between two or more atoms helps explain energetic stability and chemical reactivity. •Electrons in a single Lewis structure are insufficient to show electron delocalization. •A composite of all resonance forms more accurately depicts electron distribution. (HYBRID) NOTE: Resonance forms are not always evenly weighted. Some forms are better than others. Resonance Example •Ozone (O3) –Lewis structure of ozone shows one double bond and one single bond •• •O • Expect: one short bond and one long bond Reality: bonds are of equal length (128 pm) + O •• •• – O •• •• Resonance Example •Ozone (O3) –Lewis structure of ozone shows one double bond and one single bond •• •O • + O •• – O •• •• •• Resonance: •• •O • + O •• •• – O •• •• – •• •O • •• + O •• O •• •• Resonance Example •Ozone (O3) –Electrostatic potential map shows both end carbons are equivalent with respect to negative charge. Middle carbon is positive. •• •O • + O •• •• – O •• •• – •• •O • •• + O •• O •• •• Detailed Resonance Examples Question 7 • Which resonance structure contributes more to the hybrid? • A) B) VSEPR Model Valence Shell Electron Pair Repulsion VSEPR Model The molecular structure of a given atom is determined principally by minimizing electron pair (bonded &free) repulsions through maximizing separations. Some examples of minimizing interactions. Predicting a VSEPR Structure • 1. Draw Lewis structure. • 2. Put pairs as far apart as possible. • 3. Determine positions of atoms from the way electron pairs are shared. • 4. Determine the name of molecular structure from positions of the atoms. Orbital Geometry Chem 226 Linear Molecular Geometry Bond Angle # of lone pairs Linear 0 Trigonal Planar Trigonal Planar 0 Trigonal Planar Bent 1 Tetrahedral Tetrahedral 0 Tetrahedral Trigonal Pyramidal 1 Tetrahedral Bent 2 Trigonal Bipyramidal Trigonal Bipyramidal 0 Trigonal Bipyramidal Seesaw 1 Trigonal Bipyramidal T-shape 2 Trigonal Bipyramidal Linear 3 Octahedral Octahedral 0 Octahedral Square Pyramidal 1 Octahedral Square Planar 2 Lewis Structures / VSEPR / Molecular Models • Computer Generated Models Ball and stick models of ammonia, water and methane. Worksheet 1: Bonds, Formulas, Structures & Shapes http://chemconnections.org/organic/chem226/226assign-09.html#Worksheets http://chemconnections.org/organic/chem226/Labs/VSEPR/ Covalent Compounds •Equal sharing of electrons: nonpolar covalent bond, same electronegativity (e.g., H2) • Unequal sharing of electrons between atoms of different electronegativities: polar covalent bond (e.g., HF) Question 8 • Which of the following bonds is the most polar? • • A) B) • • C) D) Bond Dipole & Dipole Moment • Dipole moments are experimentally measured. • Polar bonds have dipole moments. dipole moment (D) = m = e x d (e) : magnitude of the charge on the atom (d) : distance between the two charges Question 9 • Which of the following bonds have the greatest dipole moment (m)? • A) B) • C) D) Bond Polarity A molecule, such as HF, that has a center of positive charge and a center of negative charge is polar, and has a dipole moment. The partial charge is represented by and the polarity with a vector arrow. H F + Question 10 • In which of the compounds below is the + for H the greatest? • A) CH4 • B) NH3 • C) SiH4 • D) H2O Question 11 • In which of the following is oxygen the positive end of the bond dipole? • A) O-F • B) O-N • C) O-S • D) O-H