PS9
advertisement

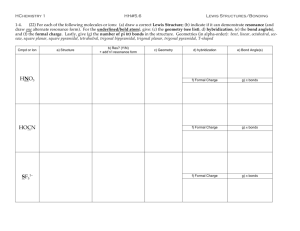
NAME_____________________________________ AP CHEMISTRY -- PROBLEM SET 9 9-1 (a) Draw the Lewis electron–dot structures for CO32- , CO2 , and CO, including resonance structures where appropriate. (b) Which of the three species has the shortest C-O bond length? Explain the reason for your answer. (c) Predict the molecular shapes for the three species. Give the hybridization scheme for each carbon. 9-2 a) Draw all possible resonance structures for the cyanate ion (OCN–) and the fulminate ion (CNO–). Put formal charges on each atom in each resonance structure. b) Use the formal charges above to predict and explain which of these two forms (fulminate or cyanate) is more stable. 9-3 Use simple structure and bonding models to account for each of the following. (a) Phosophorus forms two chlorides -- PCl3 and PCl5; nitrogen forms only NCl3 (b) The H-C-H bond angle in CH4 is 109.5 (c) The H-N-H bond angle in NH3 is 107.5. (d) The bond lengths in SO3 are all identical and are shorter than a sulfur-oxygen single bond. (e) Xenon is capable of forming compounds (e.g XeF4) while argon is not. (f) Carbon-carbon single bonds (as in ethane) rotate freely while carbon-carbon double bonds (as in ethene) do not rotate. 9-4 Draw a Lewis electron–dot structure for each of the molecules. Identify the shape and give the hybridization about the central atom. a) NO–3 b) XeF4 c) ClF3 d) NNO e) SO32– f) I–3 g) PF4– h) XeF3+ i) SF2