VIDEO 4 – SUPPORTING MATERIALS Acetylene Production
advertisement

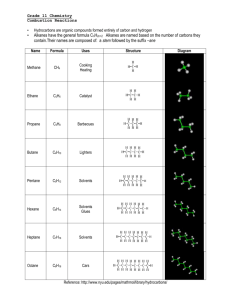
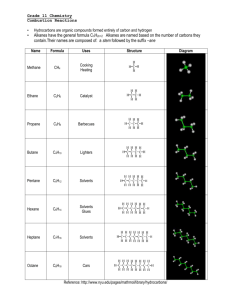
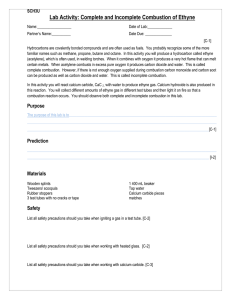
VIDEO 4 – SUPPORTING MATERIALS Acetylene Production from Calcium Carbide Summary of the Chemical Reactions The chemical equations for the reactions involved in this investigation are: CaC2(s) + 2 H2O(l) → C2H2(g) + Ca(OH)2(aq) 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g) (complete combustion) 3 C2H2(g) + 3 O2(g) → CO2(g) + CO(g) + 4 C(s) + 3 H2O(g) (incomplete combustion) Complete combustion occurs when the oxygen supply is plentiful. Incomplete combustion occurs when the oxygen supply is limited. Please note that the reaction products of incomplete combustion vary depending on the proportion of oxygen to fuel present. Hence, incomplete combustion cannot be represented by a single chemical equation. The equation provided above is only a sample equation of incomplete combustion. Prior to beginning this investigation teachers are reminded to discuss fire safety procedures with their students including: - evacuation procedures; - location and use of the fire extinguisher; - potential dangers associated with this activity; and - the need to carefully follow the instructions and example of the teacher. Safety Points • All materials should be transported on a cart with four raised sides. • The calcium carbide is water-reactive and should be kept in a sealed vial. • The splint and candle should be extinguished as soon as they are no longer required. • Safety goggles are required for this investigation. • Students must also ensure that long hair is tied back, hats removed, and loose clothing is tucked in or removed. • Students need to be a safe distance from the mouth of the test tube when blowing air gently at the flame. • Air should be blown into the test tube from the side, as opposed to from above. • You should be the only one to control the lighter and therefore when there is and is not flame in the classroom