Combustion Inquiry - Coristines
advertisement

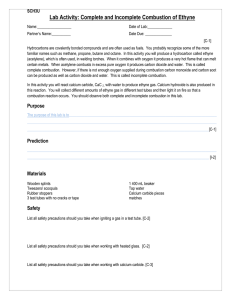
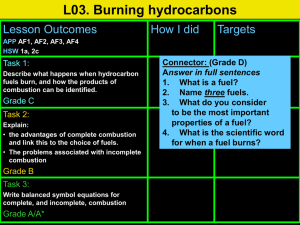
The Smoke Detector Purpose: To quantitatively demonstrate the differences between complete and incomplete combustion. Decoded: prove that changing the ratio of fuel to oxygen changes the amount o product produced. Background: You will be given a sample of calcium carbide. This mineral decomposes in water to produce acetylene gas. Acetylene gas is used as a fuel in some welding torches. Materials: Gas collection tube Splints 400 mL beaker retort stand c-clamp Hypothesis: 1) Write the balanced reaction equation for complete combustion. ____________________________________________________________ 2) Write the balanced reaction equation for incomplete combustion. ____________________________________________________________ 3) What is the ratio of fuel to oxygen in the complete vs incomplete reaction? 4) If a gas collection tube measures up to 50 mL how will you use the equation ratios to figure out how much of each of the reactants, acetylene and air, you must get into the collection tube? 5) What mass of water and soot do you expect to produce as products in each reaction? 6) Is limiting reagent an issue? Prove your statement. 7) If a complete combustion has a higher ratio of oxygen then ………….(what do you expect to see compared to an incomplete combustion………because……..(state your reasons why). Procedure: You must have carried out at least 4 variations of fuel to oxygen ratios. Thoughts: But I only calculated two in my hypothesis, what now – pick your own ratios How will you capture the acetylene? How will you determine the quantities of the reactants? How will the quantities of the reactants help determine the quantities of products? What could you measure to prove that changing the fuel/air ratio produces different amounts of products? Show your written procedure to your teacher for initialed approval. Observations: Record what you saw, smelled, measured. Discussion: 1. Was there any qualitative difference between the reactions? Explain 2. Was there a quantifiable difference between the combustion reactions? Explain with evidence. 3. What assumptions were made in this experiment that may or may not have affected the outcomes you observed?