Online Density Lab
advertisement
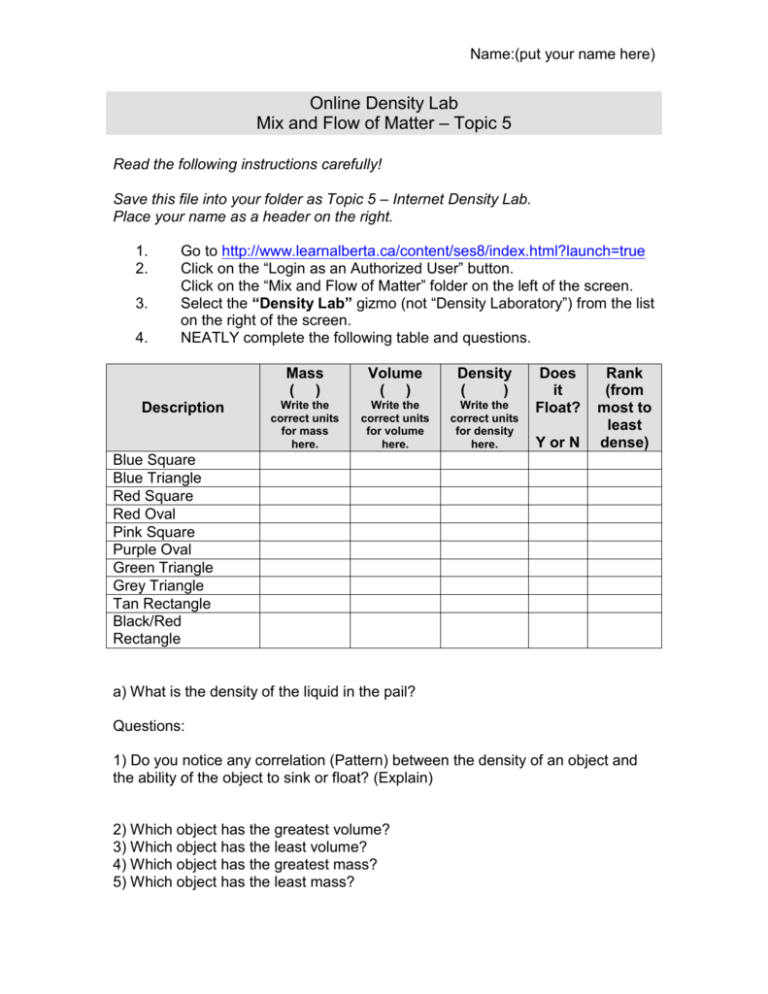
Name:(put your name here) Online Density Lab Mix and Flow of Matter – Topic 5 Read the following instructions carefully! Save this file into your folder as Topic 5 – Internet Density Lab. Place your name as a header on the right. 1. 2. 3. 4. Go to http://www.learnalberta.ca/content/ses8/index.html?launch=true Click on the “Login as an Authorized User” button. Click on the “Mix and Flow of Matter” folder on the left of the screen. Select the “Density Lab” gizmo (not “Density Laboratory”) from the list on the right of the screen. NEATLY complete the following table and questions. Description Mass ( ) Volume ( ) Density ( ) Write the correct units for mass here. Write the correct units for volume here. Write the correct units for density here. Does it Float? Y or N Rank (from most to least dense) Blue Square Blue Triangle Red Square Red Oval Pink Square Purple Oval Green Triangle Grey Triangle Tan Rectangle Black/Red Rectangle a) What is the density of the liquid in the pail? Questions: 1) Do you notice any correlation (Pattern) between the density of an object and the ability of the object to sink or float? (Explain) 2) Which object has the greatest volume? 3) Which object has the least volume? 4) Which object has the greatest mass? 5) Which object has the least mass? Name:(put your name here) 6) Which object has the greatest density? 7) Which object has the least density? 8) Which objects would sink if the density of the liquid was changed to 10g/ml? PART II Click “Back” on the website and go back to the list of Gizmos selected by you teacher. Find the “Density Experiment: Slice and Dice” Gizmo – it is the last one in the list.. Click on “Launch Gizmo.” Fill in the following table. Name of Material Styrofoam aluminum wood state Unknown A Unknown B Mass (grams) Volume (cubic cm) Sink/Float (as whole) Sink/Float (in pieces) Answers must be in bold font. 1. In your textbook, find the formula for density. 2. From your experiment, if the mass is greater than the volume of an object, does that object sink or float? 3. From your experiment, if the volume is greater then the mass of an object, does that object sink or float? 4. What is the density of the water in the gizmo? 5. For something to float, does it’s density have to be more or less than that of water? Part II 1. 2. 3. 4. 5. Highlight the final column of your table. Go to Table > Insert > Columns to the Right Title the new column “Density” Underneath “Density”, insert “g/cm3” Using the formula from question number one, calculate the density of each material (to the nearest tenth if necessary). Name:(put your name here) 6. Which substances have a density of less than 1.0? 7. Which substances float? 8. Which substances have a density of more than 1.0? 9. Which substances sink?