Study guide answer key
advertisement

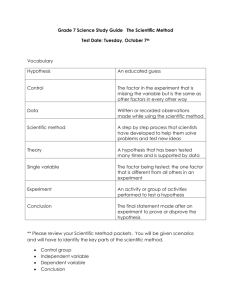
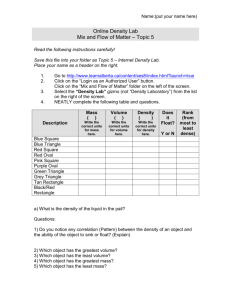
INTRO. UNIT STUDY GUIDE- test 11/13/15 For the test, you should be able to: (Check off each item you have mastered.) Demonstrate knowledge of safe laboratory rules and procedures. Demonstrate how to use various instruments to make accurate measurements. Apply correct units to all measurements. Calculate the volume of a regular solid. Calculate the volume of an irregular object, using water displacement. Calculate density, given an object’s mass and volume. Manipulate the density formula Know the density of water and why objects float or sink when placed in water. Be able to state a problem, a hypothesis and steps of the scientific method. Explain the difference between an independent and dependent variable and be able to identify them. Determine the constants in an experiment. Identify the elements of a good graph. Be able to make a conclusion after analyzing data. Calculate protons, electrons and neutrons Identify the difference between physical and chemical properties and changes For the ice/salt water lab: 1. What was the independent variable? Amount of Salt, the cause of the experiment, changed on purpose. 2. What was the dependent variable? the outcome of the lab. 3. What was the control? variable too. Melt time, The effect of the experiment, measurement for Fresh water, The factor that we compare the independent WORD BANK: balance chemicals chemical constants fire glass graduated cylinder hypothesis inference one physical problem qualitative quantitative 4. To state the __ problem is to ask a question. 5. A __ hypothesis __ is a testable statement. 6. __ constants _______ are all the things that need to stay the same during the experiment. 7. In an experiment, you can only have ____ one _____ variable. 8. A _ qualitative _______ observation is one you make with your 5 senses. 9. Measurements taken in lab are an example of _ quantitative _____ observations. 10. An __ inference _____ is an interpretation based on your knowledge and observations. 11. A ____ balance _____is used to measure mass. 12. A _ graduated cylinder ____ is used to measure volume. 13. Goggles are worn in the lab when working with __ chemicals _____, ______ fire _______________ and _______ glass ____________. 14. Burning paper is a _________ chemical ___ change. 15. Ice melting is a _____ physical ________ change. Circle C the independent variable and Underline the dependent variable for #19. 19. The higher the hikers climb in altitude, the less oxygen there is to breathe. IV: altitude DV: amount of oxygen 20. What is the formula to calculate density? D = m/v 21. Using the following information, find the density: (show your work) Mass: 30 g Volume: 15 mL D= m/v 30g/15mL = 2g/mL 22. If an object’s density is higher than water’s, it will float or sink in water. 23. If an object’s density is lower than water’s, it will float or sink in water. 24. What is the measurement for: _________29mL_________ (don’t forget your units) 25. What is the measurement of the apple in: _____________________________ (don’t forget your units) 10mL – 5mL = 5mL 26. What is the measurement for the key: ______3.3cm___________ (don’t forget your units) 27. What is the mass: __________183.6g (don’t forget your units) 28. Convert the following measurements: 204.6 cm = 0.002046 km 58.26 dg = 5826 mg 6284.25 hL = 628425 L 29. Put the scientific method in the correct order (1-7): __5___Collection of data ___1__Problem __4___Procedure __3___Materials ___2__Hypothesis __7___Conclusion __6___Analysis 30. List 4 of lab safety rules and 4 unsafe safe behaviors. 31. Identify if the following is physical or chemical change. Then explain why. Rust- Chemical change Why? When something rusts, the metal that is rusting is changed into a different substance- iron turns into iron oxide. Nail polish- Physical change Why? The nail polish is unchanged, the water it was physically mixed with evaporated. It is just coating the surface, not chemically bonding with it. Tarnish- Chemical change Why? Tarnish is a type of rust where oxygen chemically combines with silver, producing silver oxide Flammability- Chemical change Why? The material you start with, wood or paper, is changed into a new substance, ash. Element Name Element symbol Atomic # Atomic Mass Number of Protons Number of Neutrons Number of Electrons F 9 18 9 9 9 25 55 25 30 25 11 23 11 12 11 Fluorine Manganese Mn Na Sodium