Carbohydrates_2_KEY
advertisement
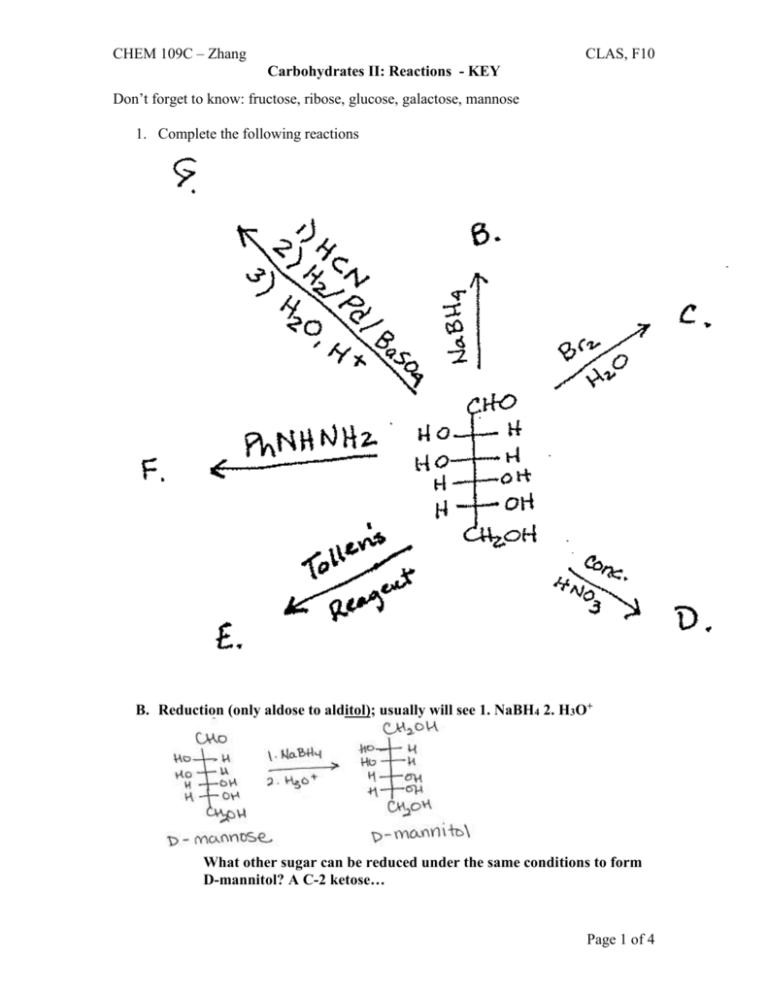
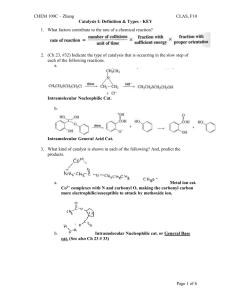
CHEM 109C – Zhang CLAS, F10 Carbohydrates II: Reactions - KEY Don’t forget to know: fructose, ribose, glucose, galactose, mannose 1. Complete the following reactions B. Reduction (only aldose to alditol); usually will see 1. NaBH4 2. H3O+ What other sugar can be reduced under the same conditions to form D-mannitol? A C-2 ketose… Page 1 of 4 CHEM 109C – Zhang CLAS, F10 Carbohydrates II: Reactions - KEY C. Oxidation (ONLY aldose to aldonic acid); loss of red-brown color of Br2 D. Strongest oxidation (aldoses, ketoses and primary OHs to form aldaric acid) E. Oxidation (BOTH aldoses and ketoses to form aldonic acid); Tollen’s reagent = Ag+, NH3, OH- F. Osazone formation; requires xs or at least 3 equivalents of phenylhydrazine C-2 epimers and the C-2 ketose will all form the same osazone Page 2 of 4 CHEM 109C – Zhang CLAS, F10 Carbohydrates II: Reactions - KEY & D-fructose G. Kiliani-Fischer synthesis; increases length of C chain by 1 C (adds a C to C1) – KNOW MECH! aldoheptoses, C-2 epimers Wohl degradation; decreases length of C chain by 1 C (removes C-1 and you lose one asymmetric center) – KNOW MECH! A. Acetal/ketal formation (hemiacetal/ketal reacting with alcohol under acidic conditions) Page 3 of 4 CHEM 109C – Zhang CLAS, F10 Carbohydrates II: Reactions - KEY 2. Label each of the following as a reducing or nonreducing sugar. Reducing Sugars Nonreducing Sugars Can reduce an oxidizing agent (Ag+ or Br2) Can NOT reduce an oxidizing agent (Ag+ or Br2) Can be oxidized Can NOT be oxidized Straight chain sugars Sugars w/out open able rings/NOT in Sugars with open able rings/in equilibrium equilibrium with the straight chain struct with the straight chain struct (hemiacetals/ketals) (acetal/ketals = glycosides, N-glycosides) A, C, F, G, H B, D, E, I, J Ch 21 #26 b. hemiacetal a. acetal c. acetal d. acetal Page 4 of 4