Catalysis 1_KEY
advertisement

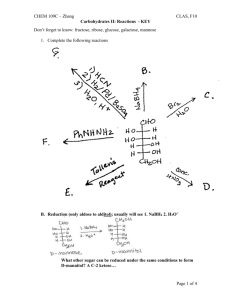
CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY 1. What factors contribute to the rate of a chemical reaction? 2. (Ch 23, #32) Indicate the type of catalysis that is occurring in the slow step of each of the following reactions. a. Intramolecular Nucleophilic Cat. b. Intramolecular General Acid Cat. 3. What kind of catalyst is shown in each of the following? And, predict the products. a. Metal ion cat. complexes with N and carbonyl O, making the carbonyl carbon more electrophilic/susceptible to attack by methoxide ion. Co2+ b. Intramolecular Nucleophilic cat. or General Base cat. (See also Ch 23 # 33) Page 1 of 6 CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY c. Intramolecular Nucleophilic cat. 4. Rank the following in order of increasing reaction rate and explain your reasoning. a. b. c. d. See Table 23.2 Uncat. rxn 6-mem ring 6-mem ring + steric hinderence keeping reactive groups close 5-mem ring 5-mem ring + steric hinderence 5-mem ring + steric hinderence Page 2 of 6 CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY 5. (Ch 23 #41) At pH = 12, the rate of hydrolysis of ester A is greater than the rate for hydrolysis of ester B. At pH = 8, the relative rates reverse (ester B hydrolyses faster than ester A). Explain these observations and show the mechanisms for the fastest hydrolysis at each pH. At pH = 12 the nucleophile is HO- and the negative charge that develops on the carbonyl O is stabilized by the N+ in ester A, so ester A reacts faster. At pH = 8 the nucleophile is water and the neutral N with its lone pair can act as a general base cat. to increase the nucleophilicity of water, so ester B reacts faster. Catalyst: A substance that increases the rate of a chemical reaction without itself being consumed or changed in the reaction. Catalysts can increase the rate of reaction by decreasing the activation energy barrier in one of three ways… a. By converting the reactant into a less stable species… __________ is the uncatalyzed pathway - - - - - - is the catalyzed pathway Ex. Specific Acid or Base Catalysis Page 3 of 6 CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY b. By providing a similar mechanism with a more stable transition state… Ex. General Acid or Base Catalysis c. By completely changing the mechanism and providing a pathway with a smaller activation energy barrier than the uncatalyzed reaction… Ex. Nucleophilic or Covalent Catalysis Types of Catalysts 1. Nucleophilic/Covalent – forms a new/covalent bond 2. Acid – donates a proton 3. Base – removes/accepts a proton Page 4 of 6 CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY Specific vs. General Acid or Base Catalysis Proton is completely transferred BEFORE the r.d.s. Occurs under strongly acidic or basic cond. Proton is transferred DURING the r.d.s. Occurs under weakly acidic or basic cond. 4. Metal Ion/Lewis Acid can increase the rate of reaction by making a better electrophile (electrophilic cat./A), making a better LG (B) or decreasing the pKa of water/making water a better Nuc (C). Similar to Table 23.1 pKas of metal-bound water. M-H2O Water/H2O Ca2+ Mg2+ Cd2+ Mn2+ Ni2+ Co2+ Zn2+ Fe2+ Cu2+ Be2+ pKa 14 12.7 11.8 11.6 10.6 9.4 8.9 8.7 7.2 6.8 5.7 5. “Intramolecular” Catalysis Page 5 of 6 CHEM 109C – Zhang CLAS, F10 Catalysis I: Definition & Types - KEY 6. Enzyme Catalyzed Reactions (see “Catalysis II handout) Examples: Decarboxylation of dimethyloxaloacetate cat. by Cu2+ or Al3+ (A) Hydrolysis of trifluoroacetate cat. by Zn2+ (C and B) Page 6 of 6