Carbohydrates_1_KEY
CHEM 109C – Zhang
Carbohydrates I: Classification and Stereochemistry - KEY
CLAS, F10
Memorize structures for: fructose, ribose, glucose, galactose, mannose
1.
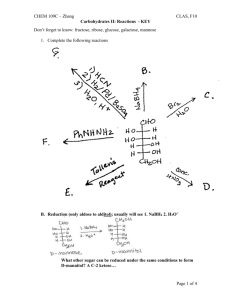
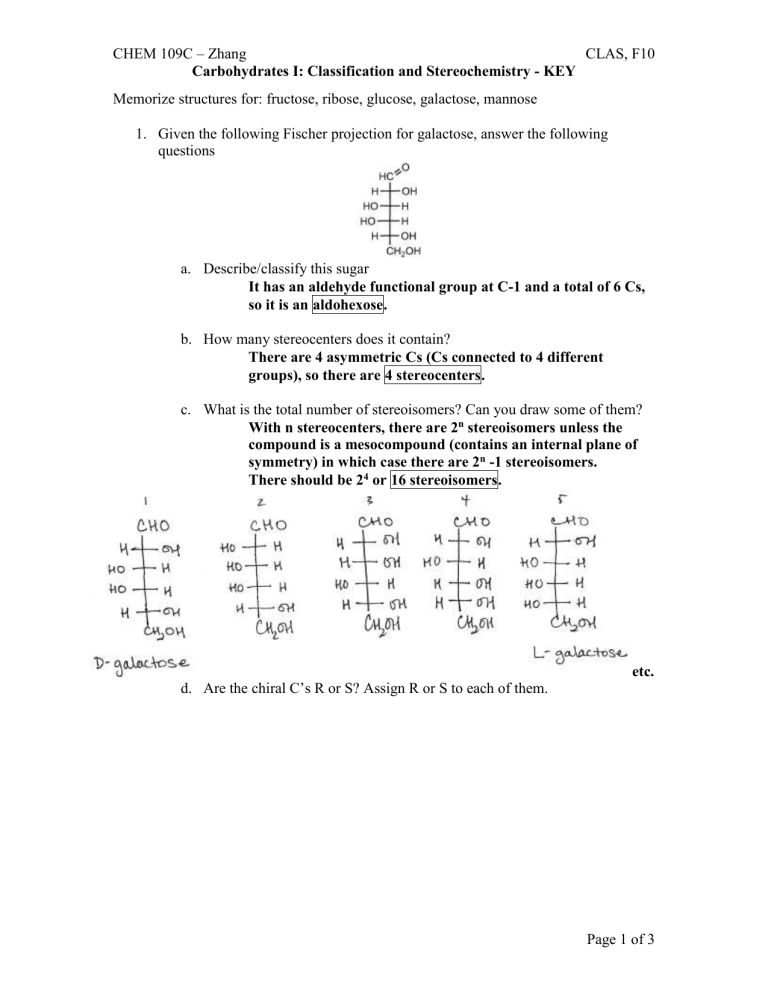
Given the following Fischer projection for galactose, answer the following questions a.
Describe/classify this sugar
It has an aldehyde functional group at C-1 and a total of 6 Cs, so it is an aldohexose. b.
How many stereocenters does it contain?
There are 4 asymmetric Cs (Cs connected to 4 different groups), so there are 4 stereocenters. c.
What is the total number of stereoisomers? Can you draw some of them?
With n stereocenters, there are 2 n stereoisomers unless the compound is a mesocompound (contains an internal plane of symmetry) in which case there are 2 n -1 stereoisomers.
There should be 2 4 or 16 stereoisomers. d.
Are the chiral C’s R or S? Assign R or S to each of them. etc.
Page 1 of 3
CHEM 109C – Zhang
Carbohydrates I: Classification and Stereochemistry - KEY
CLAS, F10 e.
Is this the D or L form?
The OH group on C-5 is on the right hand side, so this is the D form.
2.
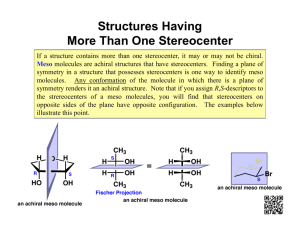
Given the carbohydrates below, answer the following questions
Page 2 of 3
CHEM 109C – Zhang
Carbohydrates I: Classification and Stereochemistry - KEY
CLAS, F10
A.
B. C. D. E.
D –glucose D-fructose D-talose L-galactose L-altrose a.
Which is a ketose sugar? B b.
Which is L-galactose? D (enantiomer of D-galactose)
The C-5 epimer of D-galactose is L-altrose (E) c.
Which is a C-4 epimer of D-galactose? A d.
Is D a C-2 epimer of D-galactose? T or F F, it is an enantiomer
The C-2 epimer of D-galactose is D-talose (C)
Page 3 of 3