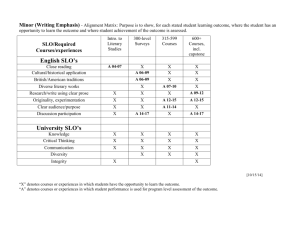
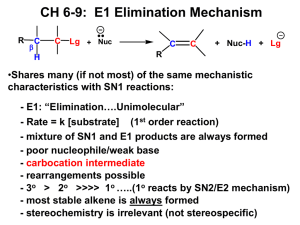
A. nitrobenzene B. bromobenzene
advertisement

1. Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the p network. 2. Provide the structure of the major organic product in the following reaction. 3.Which of the following compounds will undergo bromination most rapidly using Br2, FeBr3? A. nitrobenzene B. bromobenzene C. acetanilide D. o-methylacetanilide E. dibromobenzene 4. Rank the following groups in order of increasing activating power in electrophilic aromatic substitution reactions: -OCH3, -OCOCH2CH3, -CH2CH3, -Br. 5. Which of the following alkyl halides is most likely to undergo rearrangement in an SN1 reaction? A. 3-bromopentane B. 3-chloropentane C. 2-chloro-3,3-dimethylpentane D. bromocyclohexane E. 1-bromo-4-methylcyclohexane 6. Predict the two most likely mechanisms for the reaction of 2-iodohexane with sodium ethoxide. A. SN2 and SN1 B. E1 and E2 C. SN2 and E2 D. E1 and SN1 E. E2 and SN1 7. Predict the most likely mechanism for the reaction shown below. A. SN1 B. SN2 C. E1 D. E2 E. none of the above 8.List the following compounds in order of increasing reactivity in an SN1 reaction. CH3Br, CH3CH2CH2I, (CH3)3CI, CH3CHBrCH3, CH3CHICH3 9.Which of the following compounds will undergo an SN2 reaction most readily? A.(CH3)3CCH2I B. (CH3)3CCl C. (CH3)2CHI D.(CH3)2CHCH2CH2CH2Cl E. (CH3)2CHCH2CH2CH2I 10.Rank the species below in order of increasing nucleophilicity in hydroxylic solvents: CH3CO2-, CH3S-, HO-, H2O. 11. Which of the following is a secondary alkyl halide? A. methyl bromide B. ethyl iodide C. t-butyl iodide D. isopropyl bromide E. isobutyl chloride 12.Give the structure of the alkene which would yield the following products upon ozonolysis-reduction. CH3COCH3 + CH3CH2CHO 13.Complete the following reaction and provide a detailed, step-by-step mechanism for the process. 14.Draw the major organic product generated in the reaction below. regio- and stereochemical detail. Pay particular attention to 15.Describe a sequence of reactions by which trans-2-pentene can be straightforwardly prepared from propyne. 17. Draw an acceptable structure for a. 3,6-heptadien-1-yne b. (S)-5-phenyl-2-octyne. 18.Is it theoretically possible to separate the pair of compounds below by distillation? Explain briefly 19. Which of the following terms best describes the pair of compounds shown: enantiomers, diastereomers, or the same compound?