Name Date SN1 vs. SN2 Reactions 1) Which of the following best
advertisement

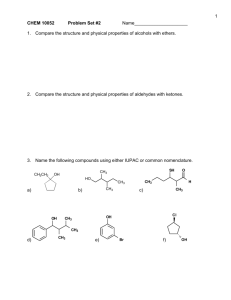
Name ______________________________________ Date __________________ SN1 vs. SN2 Reactions 1) Which of the following best represents the carbon-chlorine bond of methyl chloride? 2) Provide a detailed, stepwise mechanism for the reaction below. 1-bromo-4-methyl-pentane + KCN 3) Consider the reaction of (CH3)3CO-1 with iodomethane. Will the reaction rate increase, decrease, or remain the same if the concentration of iodomethane is increased? Explain. 4) Which of the following compounds will undergo an SN2 reaction most readily? A) C(CH3)3CH2I B) C(CH3)3Cl C) CH(CH3)2I D) CH(CH3)2CH2CH2CH2I E) CH(CH3)2CH2CH2CH2Cl 5) Would 2-chloropropane or 1-chloro-2,2-dimethylpropane undergo substitution faster with Na+ OH-1? Give the structure of the substitution product. 6) The reaction between 2-iodo-2-ethyl-hexane and ethanol to give a substitution product most likely follows an ______ mechanism. 7) Which of the following alkyl halides is most likely to undergo rearrangement in an SN1reaction? A) 3-bromopentane B) 2-chloro-2,3-dimethylpentane C) 3-chloropentane D) 2-bromo-3-methyl-hexane E) 1-bromo-4-methyl-hexane 8) Which halide has the smallest dipole moment (think about Lewis structures and partial positive and partial negative charge centers)? A) CH3F B) CH3Cl C) CH2I2 D) CH2Cl2 E) CF4 9) Arrange the substrates in order of increasing reactivity with NaCN: Bromoethane, 1-chloro-3,3-dimethyl-pentane, 2-chloro-2,2-dimethyl-pentane, and 3-bromo-3-methyl-pentane.