SN1 and SN2 and eliminations
advertisement

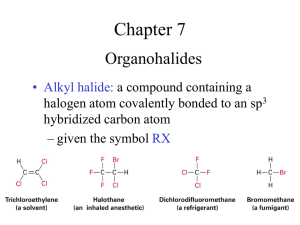
Chem 331: Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Are alkyl halides electrophiles or nucleophiles? Why? Alkyl halides react with __________ for substitutions and _______ for eliminations. What can you use a tosylate group for? Why does substitution of primary and secondary alkyl halides result in an inversion of configuration? Why doesn’t it happen for tertiary alkyl halides? What does bimolecular in SN2 represent? Fill out the table below to compare SN1 and SN2 for some quick review from lecture: SN1 SN2 Substrate Nucleophile Leaving group Solvent Can vinylic or aryl halides undergo substitution? Why or why not? How does the charge of the nucleophile affect the charge of the product? What is the strength of a nucleophile dependent on (3 things, p. 381)? What is the stability of the leaving group dependent on? Draw the intermediates for chlorination and bromination of a primary or secondary alcohol. (p.382 for help) What do these reagents do overall to speed up this reaction? Why are protic solvents bad for SN2 reactions, while aprotic solvents are the best? Why is SN1 called a unimolecular reaction? Why does the SN1 reaction produce a racemic mixture of products? When does it not produce a complete racemic mixture? Draw an allylic and benzylic carbocation intermediate. Are they more or less stable than 1° carbocations? 2°? 3°? Number the leaving groups in order of reactivity (1 is low, 6 is high): H2O___ Cl- ___ -OH___ Br-___ I-___ TosO-___ How are SN1 and SN2 affected by solvents differently? Is ethanol a better or worse solvent for SN1 reactions than water? Why doesn’t the strength of the nucleophile affect SN1 reactions very much? Elimination Reactions: What is the main difference in the three mechanisms? E1: E2: E1cB: What does cB stand for? What are the 3 pieces of evidence that support the E2 mechanism? (p. 400)