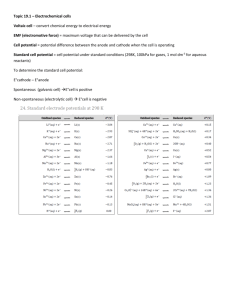
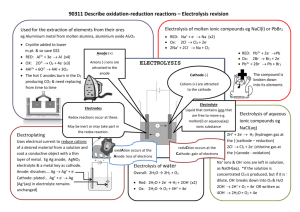
Electrochemical Cells
advertisement
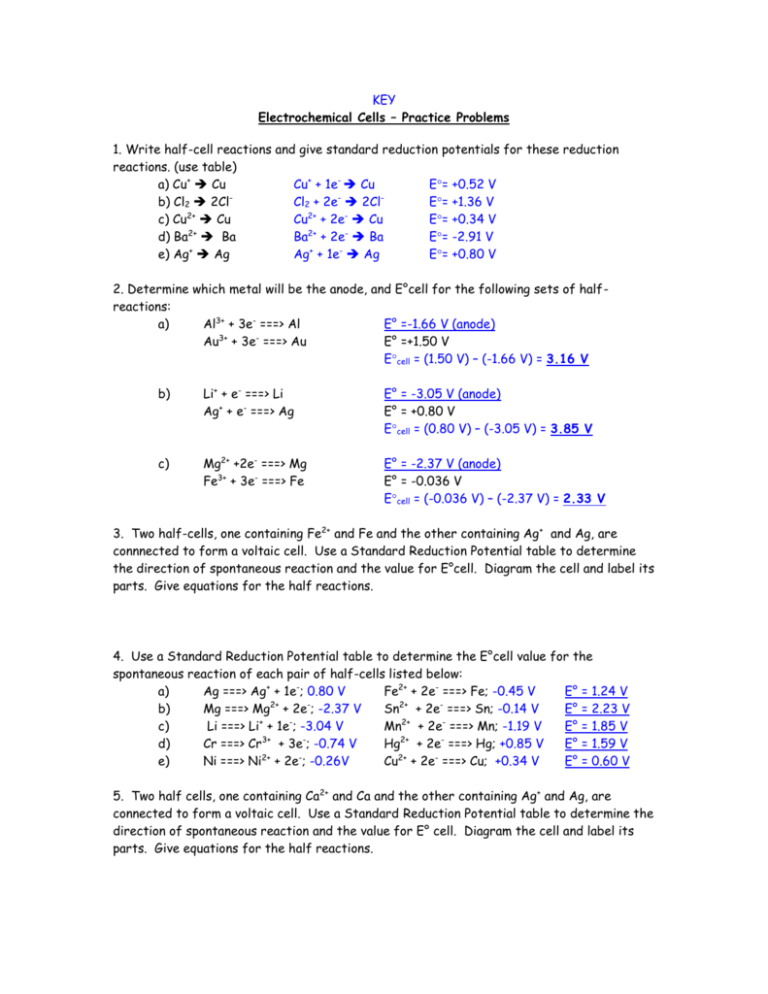
KEY Electrochemical Cells – Practice Problems 1. Write half-cell reactions and give standard reduction potentials for these reduction reactions. (use table) a) Cu+ Cu Cu+ + 1e- Cu E= +0.52 V b) Cl2 2Cl Cl2 + 2e 2Cl E= +1.36 V c) Cu2+ Cu Cu2+ + 2e- Cu E= +0.34 V 2+ 2+ d) Ba Ba Ba + 2e Ba E= -2.91 V e) Ag+ Ag Ag+ + 1e- Ag E= +0.80 V 2. Determine which metal will be the anode, and E°cell for the following sets of halfreactions: a) Al3+ + 3e- ===> Al E° =-1.66 V (anode) Au3+ + 3e- ===> Au E° =+1.50 V Ecell = (1.50 V) – (-1.66 V) = 3.16 V b) Li+ + e- ===> Li Ag+ + e- ===> Ag E° = -3.05 V (anode) E° = +0.80 V Ecell = (0.80 V) – (-3.05 V) = 3.85 V c) Mg2+ +2e- ===> Mg Fe3+ + 3e- ===> Fe E° = -2.37 V (anode) E° = -0.036 V Ecell = (-0.036 V) – (-2.37 V) = 2.33 V 3. Two half-cells, one containing Fe2+ and Fe and the other containing Ag+ and Ag, are connnected to form a voltaic cell. Use a Standard Reduction Potential table to determine the direction of spontaneous reaction and the value for E°cell. Diagram the cell and label its parts. Give equations for the half reactions. 4. Use a Standard Reduction Potential table to determine the E°cell value for the spontaneous reaction of each pair of half-cells listed below: a) Ag ===> Ag+ + 1e-; 0.80 V Fe2+ + 2e- ===> Fe; -0.45 V E° = 1.24 V 2+ 2+ b) Mg ===> Mg + 2e ; -2.37 V Sn + 2e ===> Sn; -0.14 V E° = 2.23 V c) Li ===> Li+ + 1e-; -3.04 V Mn2+ + 2e- ===> Mn; -1.19 V E° = 1.85 V 3+ 2+ d) Cr ===> Cr + 3e ; -0.74 V Hg + 2e ===> Hg; +0.85 V E° = 1.59 V e) Ni ===> Ni2+ + 2e-; -0.26V Cu2+ + 2e- ===> Cu; +0.34 V E° = 0.60 V 5. Two half cells, one containing Ca2+ and Ca and the other containing Ag+ and Ag, are connected to form a voltaic cell. Use a Standard Reduction Potential table to determine the direction of spontaneous reaction and the value for E° cell. Diagram the cell and label its parts. Give equations for the half reactions. 6. Explain why a rechargeable battery can be considered a combination voltaic-electrolytic cell. When operating in a device (calculator, phone, etc.) it is a voltaic cell (chem. energy electric energy); when being recharged, it is operating as an electrolytic cell (electric energy chem. energy). 7. A voltaic cell is composed of the following half-cells: Ca2+ + 2e- Ca E° = -2.87 V (anode) Fe3+ + e- Fe2+ E° = +0.77 V Write the reaction that takes place at the anode (oxidation) and the reaction that takes place at the cathode (reduction). Calculate the standard cell potential (E° cell). Anode: Ca Ca2+ + 2eCathode: Fe3+ + e- Fe2+ E= (0.77 V) – (-2.87 V) = +3.64 V 8. What is the standard cell potential for a voltaic cell composed of the following half-cells: Cu2+ + 2e- Cu E° = +0.34 V (anode) Ag+ + e- Ag E° = +0.80 V Write the reaction that occurs at the anode (oxidation) and the cell reaction that occurs at the cathode (reduction). Anode: Cu Cu2+ + 2eCathode: Ag+ + e- Ag E= (0.80 V) – (0.34 V) = +0.46 V 9. Is the following redox reaction spontaneous as written? (Hint: write oxidation and reduction half-reactions; look up the standard reduction potentials, and find E° cell; if E° is positive, the reaction is spontaneous!) Anode: Cathode: 2Ag+ + Ni 2Ag + Ni2+ Ni Ni2+ + 2eE= -0.26 V + 2Ag + 2e 2Ag E= +0.80 V E= (0.80 V) – (-0.26 V) = +1.06 V YES, spon. as written 10. Decide if the following redox reaction is spontaneous as written: (see hint in #9) Anode: Cathode: Cr3+ + Al Cr + Al3+ Al Al3+ + 3eE= -1.66 V Cr3+ + 3e- Cr E= -0.74 V E= (-0.74 V) – (-1.66 V) = +0.92 V YES, spon. as written