AP CHEMISTRY - Tri
advertisement

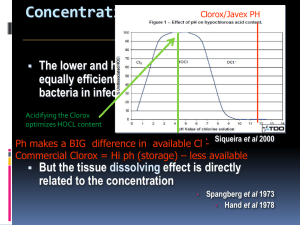
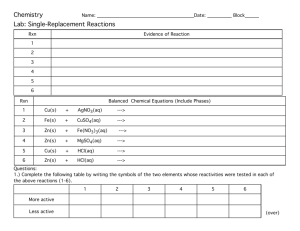
LESSON 24.3 Tri-Valley AP Chemistry ADDING STUFF* TO BUFFERS Opportunity + Dedication Success C60 (* - stuff = Strong acids and/or bases) Aside #1: Millimoles We now introduce a useful unit that you will appreciate in the next couple of lessons: the millimole. A millimole is a thousandth of a mole: 1 mmol = 0.001 mol. a) How many mmols of HOCl are in a 500. mL solution containing 0.25 M HOCl? How many moles is this? b) How many mmols of NaOCl are in a 500. mL solution containing 0.20 M NaOCL? How many moles is this? Aside #2: Completion Reactions vs. EQ Reactions When you start combining solutions, a reaction will often occur. The type of reaction depends on what’s in your solution: o OH- will always react with any acid present. The reaction will continue until one of the two (the OH- or the acid) is gone. o H+ will always react with any base present. The reaction will continue until one of the two (the H+ or the base) is gone. These reactions involving OH- and/or H+ are said to go “to completion”. For these reactions, we do NOT use EQ and ICE tables; we instead use stoichiometry and BCA (Before, Change, After) tables. Example 1. Each of the following pairs of solutions is mixed. Before any reaction occurs, what major species will be present? Identify any reactions that will go to completion. a) HCl / HNO b) HCl / NaCl c) HNO2 / HOCl MS: MS: MS: Comp. Rxn?: Comp. Rxn?: Comp. Rxn?: d) HCl / NH3 e) NaOH / HOCl MS: MS: MS: Comp. Rxn?: Comp. Rxn?: Comp. Rxn?: f) NaOH / HCl Example 2. We now add 0.05 mols of NaOH to a 500. mL solution of 0.25 M HOCl and 0.20 M NaOCl. Assume no volume change. a) Do you expect the pH of the solution to increase, decrease, or stay the same? b) What are the major species in solution before any reaction occurs? c) Will a reaction go to completion? If so, what is it? Set up the corresponding BCA table. d) What are the new major species of the solution? e) “Type of Problem”: f) What is the pH of the resulting solution? Example 3. To your original solution of 500. mL 0.25 M HOCl and 0.20 M NaOCl, you add 0.05 mols HCl. Assume no volume change. a) Do you expect the pH of the solution to increase, decrease, or stay the same? b) What are the major species in solution before any reaction occurs? c) Will a reaction go to completion? If so, what is it? Set up the corresponding BCA table. d) What are the new major species of the solution? e) “Type of Problem”: f) What is pH of the resulting solution?