Pre-Lab for Basics of Titrations Laboratory

Pre-Lab for Basics of Titration Laboratory
The procedure for the laboratory can be found at the link below. Make sure you print a copy of the procedure in addition to the pre-lab assignment. The pre-lab quiz for this week is to complete the assignment on this page BEFORE your laboratory starts. This assignment replaces the normal prelab quiz and will help you complete your laboratory experiment on time. http://www.cord.edu/faculty/dmork/chem128L/TitrationExpt.doc
1.
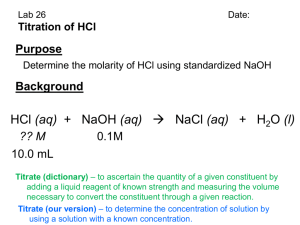
Determine how you will prepare the NaOH and HCl stock solutions for this week’s laboratory.
Write the procedure that you plan to use in lab in the space below. Include exact measurements of concentrated NaOH and HCl that you will use. The procedure you write below should have enough detail so that your laboratory instructor could prepare the solutions from your directions.
Procedure for preparing NaOH solution:
Procedure for preparing HCl solution:
2.
If you used 17.65 mL of 0.138 M NaOH to titrate 10.00 mL of a dilute HCl solution, what is the concentration of the dilute HCl?
Answer: ____________________
3.
If you anticipate that your HCl solution will be around 0.200 M, who many milliliters of 0.140 M
NaOH will you need to titrate 10.00 mL of the HCl solution?
Answer: ______________________