Electron config worksheet
advertisement

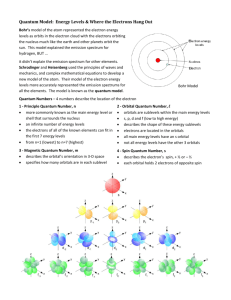
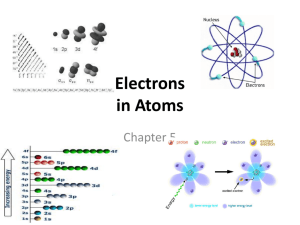
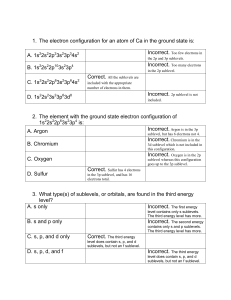
Name_________________________ Hour_________________________ Electron Configuration Worksheet 1. Use the periodic table to write the ground state electron configuration for the following: a. Si ___________________________________________________________________ b. Se ___________________________________________________________________ c. Rb ___________________________________________________________________ d. I _____________________________________________________________________ 2. Write the outer shell configuration for the following: a. nitrogen ________________________ b. phosphorus _________________________ c. oxygen _________________________ d. sulfur ______________________________ e. argon ___________________________ e. xenon ______________________________ The chemical properties of atoms, ions and molecules are related to the arrangement of the (3) ________________________ within them. The first modern atomic theory, proposed by (4) __________________, portrayed the atoms as a solid, indivisible mass. After the discovery of the electron by (5) ________________, the atomic model was revised to include them. Rutherford pictured the atom having a dense (6)____________________ surrounded by electrons. In the Bohr model, the electrons move in set (7) ______________________. The (8) _____________________ model is the modern description of the electrons in atoms. This model estimates the (9) ________________________ of finding an electron within a certain volume of space surrounding the nucleus. True or False _____ 10. The number of sublevels in an energy level is equal to the square of the principle quantum number of that energy level. _____ 11. The maximum number of electrons that can occupy the fourth principle energy level of an atom is 32. _____ 12. The principle quantum number equals the number of sublevels within that principle energy level. _____ 13. The position and velocity of an electron in an atom can be determined with great certainty. _____ 14. The photoelectric effect will occur no matter what frequency of light strikes a metal. 1 The ways in which electrons are arranged around the nuclei of atoms are called (15)____________________________________. The (16) ___________________________ describes the sequence in which orbitals are filled. The various orbitals within a sublevel of a principle energy level are always of (17) _____________ energy. The (18) ______________ _____________________________ principle states that a maximum of only (19) __________ electrons can occupy each orbital. To occupy the same orbital two electrons must have (20)_______________ spins. Hund’s rule states that the electrons pair up only after each orbital in a sublevel is occupied by (21) ________________________. For showing the electron configuration of an atom, (22) ___________________ are used to indicate the number of (23)________________ occupying each sublevel. According to quantum mechanics, the motions of subatomic particles may be described as (24) ______________________. Every element emits (25) _________________________ if it is heated by passing an electric discharge through its gas or vapor. 26. How many s orbitals are there in each principal energy level? ________________________ 27. How many orbitals are there in the n=4 principal energy level? _______________________ 28. How many electrons can the n=4 principal energy level hold maximum? ________________ 29. How many orbitals are in each of the following: 4p sublevel _______; 3d sublevel________; 4f sublevel _______; 2s sublevel ________ 30. Which is the lowest atomic number element with a full third energy level? _______________ 31. Which is the lowest atomic number element that contains a p electron? __________________ 32. How many sublevels are in the following principal energy levels? n=1_______; n=2_______; n=3_______; n=4 _________. 33. Write an electron configuration for the following: a. barium ______________________________________________________________________ b. krypton _____________________________________________________________________ c. bromine _____________________________________________________________________ d. tellurium ____________________________________________________________________ 34. Give the number of protons, neutrons, and electrons in each of the following: a. 21Na ___________________________________ b. 81Br_____________________________ c. 235U____________________________________ d. 41Ca_____________________________ 2