Quantum Numbers and Lewis Dots for Atoms worksheet
advertisement
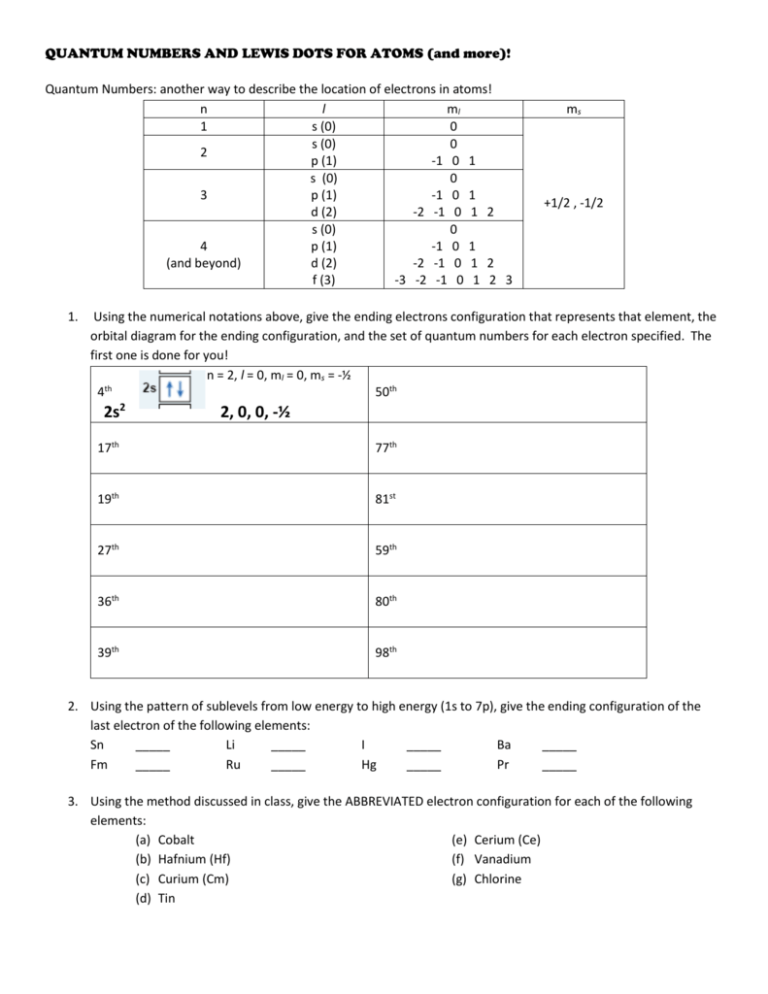
QUANTUM NUMBERS AND LEWIS DOTS FOR ATOMS (and more)! Quantum Numbers: another way to describe the location of electrons in atoms! n l ml 1 s (0) 0 s (0) 0 2 p (1) -1 0 1 s (0) 0 3 p (1) -1 0 1 d (2) -2 -1 0 1 2 s (0) 0 4 p (1) -1 0 1 (and beyond) d (2) -2 -1 0 1 2 f (3) -3 -2 -1 0 1 2 3 1. ms +1/2 , -1/2 Using the numerical notations above, give the ending electrons configuration that represents that element, the orbital diagram for the ending configuration, and the set of quantum numbers for each electron specified. The first one is done for you! n = 2, l = 0, ml = 0, ms = -½ th 4 50th 2s2 2, 0, 0, -½ 17th 77th 19th 81st 27th 59th 36th 80th 39th 98th 2. Using the pattern of sublevels from low energy to high energy (1s to 7p), give the ending configuration of the last electron of the following elements: Sn _____ Li _____ I _____ Ba _____ Fm _____ Ru _____ Hg _____ Pr _____ 3. Using the method discussed in class, give the ABBREVIATED electron configuration for each of the following elements: (a) Cobalt (e) Cerium (Ce) (b) Hafnium (Hf) (f) Vanadium (c) Curium (Cm) (g) Chlorine (d) Tin QUANTUM NUMBERS AND LEWIS DOTS FOR ATOMS (and more)! 4. Tell whether the following are in GROUND state (GS), EXCITED state (ES) or IMPOSSIBLE (I) electron configurations. (a) 1s22s22p4____ (e) 1s22s22p10____ (b) 1s22s22p63p1 ____ (f) [Ar]3s2____ (c) 1s22s22p63s23p64s23d84p1____ (g) 1s22s22p63s33p64s23d104p65s1 ____ 1 (d) [Xe]7s ____ 5. List the maximum number of electrons possible in each of these “spaces”: a. a p sublevel __________ b. a 3d orbital _________ c. the 4th energy level _________ d. the 1st energy level _________ e. the 5f sublevel ___________ f. a 7s orbital ___________ g. the 7th energy level ___________ h. the 5d sublevel ____________ 6. List the number of valence electrons in each element, and draw the Lewis dot diagram that represents them around the symbol for the element: a) Si i) Ra b) Kr j) P c) Sr k) Rn d) Co l) H e) Na m) W f) Al n) Sn g) Te o) Cl h) Cd p) Fr