THE REDUCTION OF A CARBONYL WITH SODIUM
advertisement

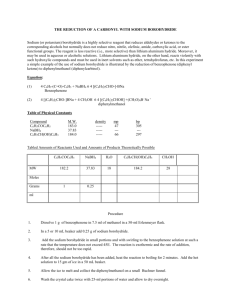
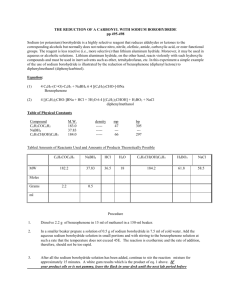
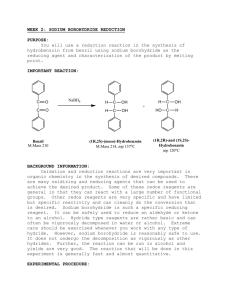
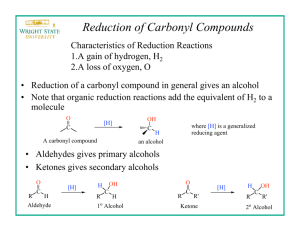
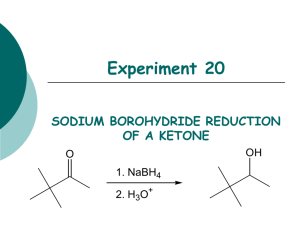
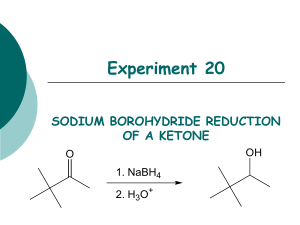
THE REDUCTION OF A CARBONYL WITH SODIUM BOROHYDRIDE Sodium (or potassium) borohydride is a highly selective reagent that reduces aldehydes or ketones to the corresponding alcohols but normally does not reduce nitro, nitrile, olefinic, amide, carboxylic acid, or ester functional groups. The reagent is less reactive (i.e., more selective) than lithium aluminum hydride. Moreover, it may be used in aqueous or alcoholic solutions. Lithium aluminum hydride, on the other hand, reacts violently with such hydroxylic compounds and must be used in inert solvents such as ether, tetrahydrofuran, etc. In this experiment a simple example of the use of sodium borohydride is illustrated by the reduction of benzophenone (diphenyl ketone) to diphenylmethanol (diphenylcarbinol). Equation: (1) 4 C6H5-(C=O)-C6H5 + NaBH4 6 4 [(C6H5)2CHO-]-BNa Benzophenone (2) 4 [(C6H5)2CHO-]BNa + 4 CH3OH 6 4 [(C6H5)2CHOH] +(CH3O)4B- Na + diphenylmethanol Table of Physical Constants Compound M.W. density mp bp C6H5COC6H5 183.0 ----- 47 305 NaBH4 37.83 ----- --- --- C6H5CH(OH)C6H5 184.0 ----- 66 297 Tabled Amounts of Reactants Used and Amounts of Products Theoretically Possible MW C6H5COC6H5 NaBH4 H2O C6H5CH(OH)C6H5 CH3OH 182.2 37.83 18 184.2 28 1 0.25 Moles Grams ml Procedure 1. 2. Dissolve 1 g of benzophenone in 7.5 ml of methanol in a 50-ml Erlenmeyer flask. In a 5 or 10 mL beaker add 0.25 g of sodium borohydride. 3. Add the sodium borohydride in small portions and with swirling to the benzophenone solution at such a rate that the temperature does not exceed 45E. The reaction is exothermic and the rate of addition, therefore, should not be too rapid. 4. After all the sodium borohydride has been added, heat the reaction to boiling for 2 minutes. Add the hot solution to 15 gm of ice in a 50 mL beaker. 5. Allow the ice to melt and collect the diphenylmethanol on a small Buchner funnel. 6. Wash the crystal cake twice with 25-ml portions of water and allow to dry overnight. 7. At the beginning of the next lab period, weigh the product, and determine its melting point. Yield about 1 g (95%). Include in your notebook and in your Lab Report, also calculate the % yield. 7. Use this product in the alcohol classification assignment.