- Ellis Benjamin

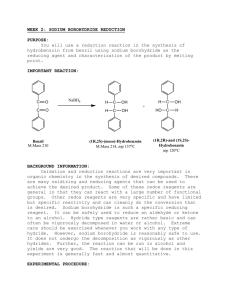
Procedure for Sodium Borohydride Reduction
To a reaction tube, add 1.25 mL of methanol and 300 mg of 2-methyl cyclohexanone. Cool this solution in an ice bath contained in a small beaker.
While the reaction tube is in the ice bath, carefully add 50 mg of sodium borohydride to the solution. After the vigorous reaction has ceased, remove the tube from the ice bath and allow it to stand at room temperature for 10 min, at which time the reaction should appear to be finished. To decompose the borate ester add 1.25 mL of 3 M sodium hydroxide solution. To the resulting cloudy solution, add 1 mL of water. The product will separate as a small, clear upper layer. Remove as much of this as possible, place it in a reaction tube, and then extract the remainder of the product from the reaction mixture with two 0.5-mL portions of dichloromethane. Add these dichloromethane extracts to the small product layer and dry the combined extracts over anhydrous sodium sulfate (not calcium chloride). After a few minutes, transfer the solution to a dry reaction tube containing a boiling chip. In the hood, boil off the dichloromethane (and any accompanying methanol) and use the residue to run an NMR spectrum in the usual way.
Integrate the two low-field sets of peaks at 3.1 and 3.8 ppm, analyze the coupling constant patterns to assign the two sets of peaks, and report the percentage distribution of cis and trans -2-methylcyclohexanol formed in this reaction. The relative amounts of the products can, of course, also be determined by gas chromatography. How do these results compare to your predictions and calculations? Which cis and which trans conformation is the more stable?
Run an infrared (IR) spectrum of the product as a thin film to determine whether the reduction of the starting material has been completed (Fig. 26.2).
Cleaning Up.
The reaction mixture is neutralized with acetic acid (to react with sodium borohydride) and flushed down the drain with water.