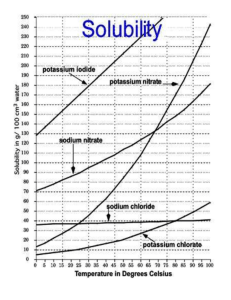
and sodium chlorate(V)
advertisement
77 Potassium chlorate(V) & chlorate(VII) and sodium chlorate(V) Do NOT make mixtures of potassium chlorate(V) or sodium chlorate (V) with sulfur and/or phosphorus. Potassium chlorate(V) Potassium chlorate(VII) Sodium chlorate(V) Oxidising O Harmful Store: Ox H KClO3 KClO4 NaClO3 R9: Explosive when mixed with combustible material. R20/22: Harmful by inhalation and if swallowed. R51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Dangerous with: AMMONIA and AMMONIUM SALTS. Unstable, explosive salts form even when mixed dry. METAL POWDERS, MANGANESE(VI) OXIDE, SULFIDES, THIOSULFATES, CARBON, SUGARS, HYDROCARBONS. Unstable & dangerous mixtures are formed. CONCENTRATED SULFURIC(VI) ACID and PHOSPHORIC(V) ACID. Explosive chlorine oxides or chloric acids are produced. COMBUSTIBLE MATERIALS: SUGAR, WOOD, CLOTHES etc, impregnated with solutions, ignite easily and burn very vigorously when dry. These reactions may be carried using special risk assessments (eg, the howling jelly-baby demonstration). Emergencies: see standard procedures on Hazcard E, BUT ALSO: If spilt in the laboratory: It is essential to ensure that no significant impregnation of wood, cloth or other combustible matter occurs. Never use fabric gloves to handle these substances. Disposal: W1, Wspec -3 Wspec: Dilute to, or prepare, a 0.1 mol dm solution. Add about 10 g of sodium or 3 potassium metabisulfite to 100 cm of this solution. Leave for 1 hour in a fume cupboard. Pour the solution down a foul-water drain. Potassium perchlorate Potassium chlorate(V) and sodium chlorate(V) should not be available in the laboratory at the same time as concentrated sulfuric(VI) acid. Do not confuse sodium chlorate(V) (NaClO3), a commercial weed killer, usually found as a solid, with sodium chlorate(I) (NaClO), a disinfectant, which is only found as a solution. Where chlorates are required by a scheme of work, potassium chlorate(V) usually satisfies the requirements and there is little need to keep sodium chlorate(V). For emergencies, see Hazcard E as well as more detailed information which may be on this Hazcard. © CLEAPSS 2009 77 Potassium chlorate(V) & chlorate(VII) and sodium chlorate(V) Model risk assessments Activity Solubility investigations using potassium chlorate(V) User Y9 Control measures Wear eye protection. Take care not to allow the solution to impregnate wood or clothing. Redox reactions Action of heat Y12 Wear eye protection. Y12 Wear goggles. Do not add manganese(IV) oxide to catalyse the reaction. Catalysis of the decomposition of potassium chlorate(V) TT Wear goggles or a face shield. Preparation of oxygen using chlorates Howling jelly-baby demonstration TT There are better ways of producing oxygen. Do not use this method. Wear goggles or a face shield. Use safety screens. TT Experimental points 3 The solubility changes from 7.1 g to 57 g per 100 cm of water in the temperature range 20 to 100 °C, making this an ideal chemical to investigate solubility changes with temperature. Collect all solutions at the end for disposal. Some courses suggest this activity for younger pupils and, with well-motivated pupils, this may be possible. Aluminium potassium sulfate(VI) and sodium tetraborate (anhydrous) are safer alternatives. -3 Work on a test-tube scale. Use 0.1 mol dm solutions to oxidise iron(II) or iodide ions. Use 1-2 g in a dry, borosilicate test tube. Place a mineral-wool plug in the neck of the test tube; this stops the student allowing the glowing splint to touch the molten salt. Under careful conditions, potassium chlorate(VII) can be made and isolated. These crystals should not be stored but immediately disposed of after isolation and weighing. ‘Oxygen mixture’, ie, potassium chlorate(V) and manganese(IV) oxide, should not be stored nor used for the preparation of oxygen (see below). A mixture can be made to demonstrate the catalytic activity of oxides using about 0.3 g of potassium chlorate(V) and a pinch of manganese(IV) oxide or copper(II) oxide in an ignition tube. Zinc oxide or magnesium oxide could be used to show that they have no effect. Numerous accidents have been reported with the traditional method of heating potassium chlorate(V) with manganese(IV) oxide. See Hazcard 69 for the preparation of oxygen. The supplementary risk assessment for this demonstration is available on the CLEAPSS members-only web site and Science Publications CD-ROM. For emergencies, see Hazcard E as well as more detailed information which may be on this Hazcard. © CLEAPSS 2007