Prelab Questions: The Composition of Potassium Chlorate
advertisement

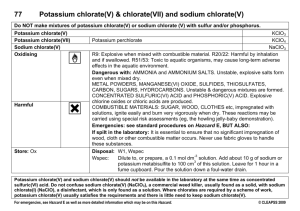
Prelab Questions: The Composition of Potassium Chlorate 1) In Part A of this lab, you will analyze a sample of potassium chlorate. What is the chemical formula of potassium chlorate? 2) The goal of this analysis is to experimentally to determine the mass percent of one of the elements in potassium chlorate. Identify this element. 3) To perform the analysis, you will decompose the potassium chlorate by heating it to a high temperature. What are the products of this decomposition reaction? Can you write the balanced equation for this reaction? 4) The potassium chlorate sample will be heated in a specialized "container". a) What is this container called? b) Will this container be covered or uncovered? c) What other equipment will you use? Be sure to look at photos of this equipment so that you can easily identify them in lab! 5) What is the minimum number of times that your sample of potassium chlorate must be heated (1, 2, 5 etc.)? What is the minimum amount of time that this will take (6 minutes, 30 minutes, etc.)? Will you heat it at a high temperature, low temperature or both? If both, in which order? 6) Since Part A involves a quantitative analysis of potassium chlorate, you will be performing several mass measurements throughout the lab. a) What exactly will you be weighing? b) What type of balance will you use, and what are the units of your mass measurements? c) What are two precautions you must observe when using the balance? 7) Identify the substance that will be left in the "container" after heating is completed (the "residue"). Do you expect it weigh more than, less than or the same as the original potassium chlorate sample? Why? 8) Exactly how will you use your experimental results to obtain the mass percent of the "element of interest" in this experiment? What "formula" will you use for the calculation? 9) In Part B of this lab, you will analyze the residue in the "container" in order to prove its identity. To do this, you will need three medium test tubes, labeled tube #1, tube #2 and tube #3. a) What substances will be added to each of these test tubes? b) What chemicals (2 total) will then be added to each of these substances to test them? c) What will you see if you obtain a positive test for chloride ions? 10) What are two important safety issues specific to this lab?