SCH3U Composition of Substances 2
advertisement

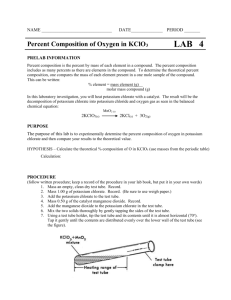
SCH3U1 The Composition of Substances 2 1.122.5 g of potassium chlorate is composed of 39.0 g potassium, 35.5 g of chlorine and 48.0 g of oxygen. Calculate the % of each element in the compound. 2. A compound with a mass of 75.0 g was found to contain 52% carbon. 13% hydrogen and 35% oxygen. What was the mass of each element in the compound? 3. 8.00 g of copper oxide is heated with 1.0 g of carbon. The products are carbon dioxide and copper. a) If 6.40 g of copper are formed, what mass of carbon dioxide is produced? b) What mass of oxygen is present in the copper oxide that is formed? c) What is the % copper and % oxygen in the copper oxide? 4. 8.0 g of copper oxide react with 0.2 g of hydrogen to form 1.8 g of water and copper. a) What mass of copper is formed? b) Calculate the % of hydrogen and % oxygen in water. 5. A compound contains 36.5 % sulfur. How much sulfur could be extracted from 2000 g of this compound? 6. Calculate the mass percent of nitrogen in each formula. a) N2O b) Sr(NO2)2 c) NH4NO3 d) HNO3 7. The mineral pyrolusite is composed of manganese oxide, MnO2. a) Calculate the percent composition of MnO2. b) What mass of pure manganese can be extracted from 500 kg of pyrolusite? 8. What is the percent composition of sulfuric acid, H2SO4? 9. A test tube containing potassium chlorate was heated until no more oxygen was evolved. The other product left in the test tube was potassium chloride. a) From the given experimental data, calculate the % oxygen in potassium chlorate. mass of empty test tube…………………………………………..18.00 g mass of test tube + potassium chlorate…………………………24.13 g mass of test tube and potassium chloride………………………21.73 g b) Calculate the theoretical value for the % oxygen in potassium chlorate (KClO3). 10. Indigo (C16H10N2O2) is the common name for the blue dye used in jeans. Calculate the mass of oxygen in 25.0 g of indigo. 11. Determine the % composition of acetaminophen, the active ingredient in many analgesic (pain relieving) drugs such Tylenol, given that it has the chemical formula C8H9NO2 (shown at right). Answers: 1 31.8% K; 29.0% Cl; 39.2% O 2. 39g C; 10g H; 26 g O 3a) 2.60 g; b) 1.60 g; c) 80.0% Cu; 20.0% O 4a) 6.4 g; b) 11% H; 89% 5. 7.3 x 102 g 6 a) 63.65% N b) 15.60% N c) 35.00% N d) 22.23% N 7. a) 63.19% Mn; 36.81% O b) 316 kg 8. 2.06% H; 32.69% S; 62.25% O 9a) 39.2%; 39.2% 10.3.05 g 11. 63.5%C; 6.02% H; 9.27%N; 21.2% O