Nomeclature Note 1.wpd
advertisement
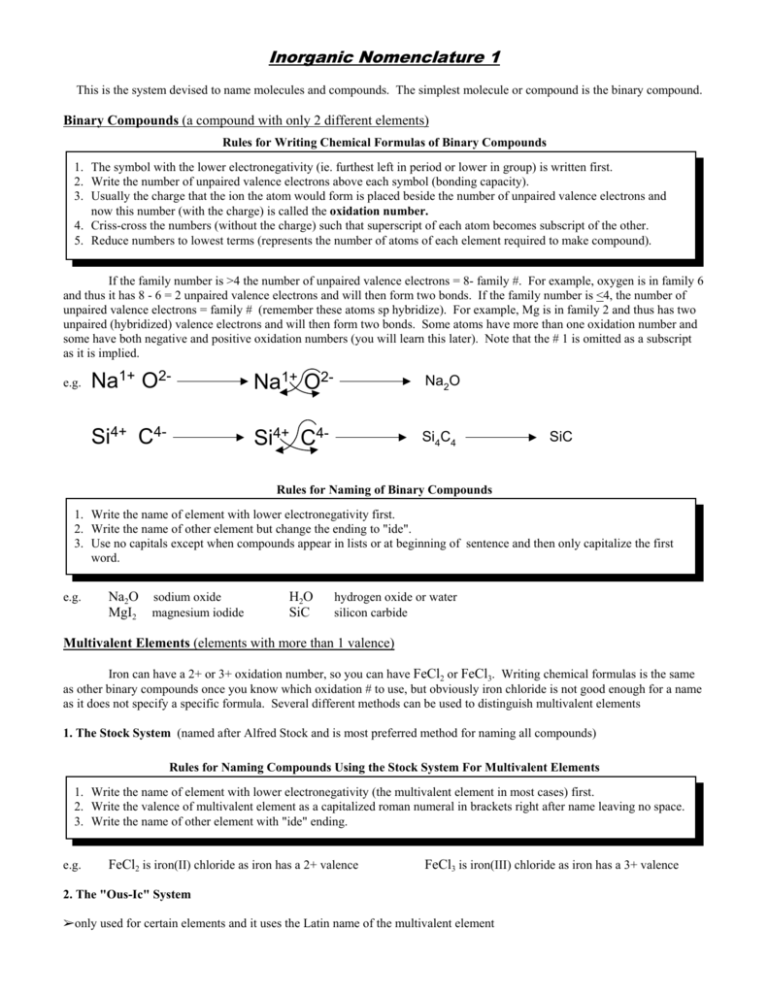
Inorganic Nomenclature 1 This is the system devised to name molecules and compounds. The simplest molecule or compound is the binary compound. Binary Compounds (a compound with only 2 different elements) Rules for Writing Chemical Formulas of Binary Compounds 1. The symbol with the lower electronegativity (ie. furthest left in period or lower in group) is written first. 2. Write the number of unpaired valence electrons above each symbol (bonding capacity). 3. Usually the charge that the ion the atom would form is placed beside the number of unpaired valence electrons and now this number (with the charge) is called the oxidation number. 4. Criss-cross the numbers (without the charge) such that superscript of each atom becomes subscript of the other. 5. Reduce numbers to lowest terms (represents the number of atoms of each element required to make compound). If the family number is >4 the number of unpaired valence electrons = 8- family #. For example, oxygen is in family 6 and thus it has 8 - 6 = 2 unpaired valence electrons and will then form two bonds. If the family number is <4, the number of unpaired valence electrons = family # (remember these atoms sp hybridize). For example, Mg is in family 2 and thus has two unpaired (hybridized) valence electrons and will then form two bonds. Some atoms have more than one oxidation number and some have both negative and positive oxidation numbers (you will learn this later). Note that the # 1 is omitted as a subscript as it is implied. e.g. Na1+ O2- Na1+ O2- Na2O Si4+ C4- Si4+ C4- Si4C4 SiC Rules for Naming of Binary Compounds 1. Write the name of element with lower electronegativity first. 2. Write the name of other element but change the ending to "ide". 3. Use no capitals except when compounds appear in lists or at beginning of sentence and then only capitalize the first word. e.g. Na2O sodium oxide MgI2 magnesium iodide H2O SiC hydrogen oxide or water silicon carbide Multivalent Elements (elements with more than 1 valence) Iron can have a 2+ or 3+ oxidation number, so you can have FeCl2 or FeCl3. Writing chemical formulas is the same as other binary compounds once you know which oxidation # to use, but obviously iron chloride is not good enough for a name as it does not specify a specific formula. Several different methods can be used to distinguish multivalent elements 1. The Stock System (named after Alfred Stock and is most preferred method for naming all compounds) Rules for Naming Compounds Using the Stock System For Multivalent Elements 1. Write the name of element with lower electronegativity (the multivalent element in most cases) first. 2. Write the valence of multivalent element as a capitalized roman numeral in brackets right after name leaving no space. 3. Write the name of other element with "ide" ending. e.g. FeCl2 is iron(II) chloride as iron has a 2+ valence FeCl3 is iron(III) chloride as iron has a 3+ valence 2. The "Ous-Ic" System %only used for certain elements and it uses the Latin name of the multivalent element Rules for Naming Compounds Using the Ous-Ic System For Multivalent Elements 1. Write the Latin name of first (multivalent) element with suffix "ous" added if it has the lower valence and "ic" if it has the higher valence. 2. Write the name of other element with "ide" ending. e.g. FeCl2 is ferrous chloride as iron has a 2+ valence FeCl3 is ferric chloride as iron has a 3+ valence Here are the Latin names and valences you have to know. English Name Latin Name Valences Number Prefix Number Prefix iron ferrum 2,3 1 mono 5 penta copper cuprum 1,2 2 di 6 hexa lead plumbum 2,4 3 tri 7 hepta tin stannum 2,4 4 tetra 8 octa mercury mercurum 1,2 3. The Prefix System (typically non-metal bonded to another non-metal) Rules for Naming Molecules Using the Prefix System For Multivalent Elements 1. Add the appropriate prefix to name of either the first, second or both element to indicate the number of atoms of that element present in compound. 2. Write the name of second element with "ide" ending. e.g. e.g.2. CO carbon monoxide N2O4 dinitrogen tetroxide CO2 carbon dioxide N.B. mono is never used for the first element N.B. if the second element is oxygen and prefix for oxygen ends in "a" or "o", the "o" or "a" is dropped Prepared by K. Zuber







