Valence Electron Practice Worksheet
advertisement

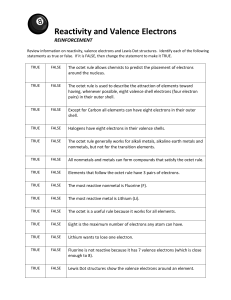
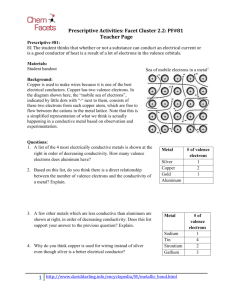
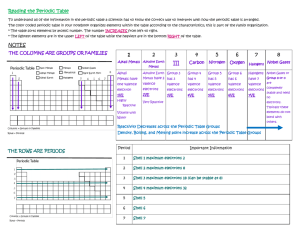
Valence Electron Practice Name: ___________________________________________________ Period: _______ 1. What is a valence electron and why are they important to a chemist? 2. How can you find the number of valence electrons an element has? 3. How many protons are in the following elements? A. Carbon B. Hydrogen C. Oxygen D. Phosphorus E. Calcium 4. Draw the atomic structures for the following. Include the number of protons, neutrons and draw in the valence electrons. A. Silicon B. Potassium C. Sulfur D. Beryllium E. Argon F. Nitrogen 5. Indicate whether the following elements will GAIN or LOSE electrons to get a full outer shell, and how many electrons they will need to GAIN or LOSE. Example: Chlorine has 7 outer shell electrons, so it will GAIN 1 electron to get a full outer shell. A. Lithium C. Calcium E. Boron G. Tin B. Bromine D. Oxygen F. Argon H. Phosphorus 6. What will the charge be of the following element when they GAIN or LOSE electrons to become stable? A. Aluminum C. Nitrogen E. Sulfur G. Magnesium B. Iodine D. Potassium F. Neon H. Carbon 7. Write the symbol and the charge for the atoms given below and identify the element as a cation/anion and metal/nonmetal. Element Calcium Bromine Nitrogen Fluorine Aluminum Phosphorus Lithium Symbol Charge Metal or Nonmetal Anion or Cation