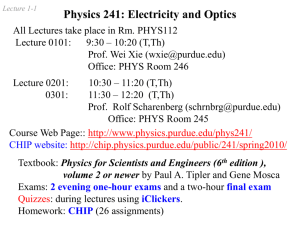
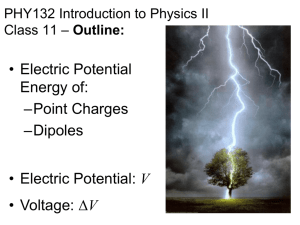
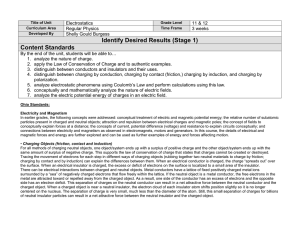
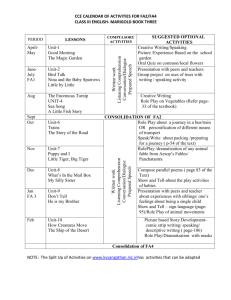
Lewis structure replacement quiz
advertisement

Draw the following Lewis structures with correct shapes. Label all shapes. Show all lone pair and formal charges. If resonance structures exist for a given molecule, show all of them. A substantial amount of credit will be forfeit if any of these requirements are omitted. ***For #9, use formal charges to show why N is the central atom and not O. Important! Your answers to this quiz must be submitted by 1:00pm on 2/24/15. A basket will be available outside of my office (Sims 302B). No late submissions will be allowed. If you did not earn at least an 80% on the original quiz, you are required to submit this replacement quiz. Failure to do so will result in a grade of ZERO. 1. 2. 3. 4. 5. 6. 7. 8. 9. Xenon Dioxide Bromine pentafluoride phosphate sulfur trioxide AsO3Krypton tetrachloride NO2F N3nitrate