9.3: PREDICTING REDOX REACTION
advertisement
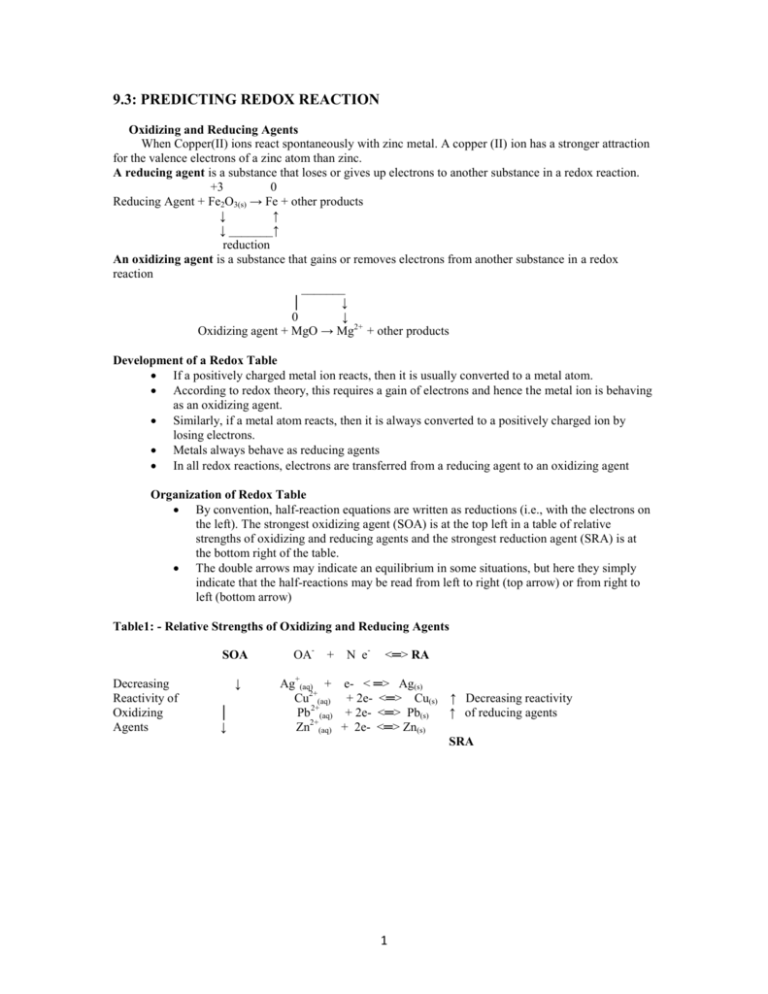
9.3: PREDICTING REDOX REACTION Oxidizing and Reducing Agents When Copper(II) ions react spontaneously with zinc metal. A copper (II) ion has a stronger attraction for the valence electrons of a zinc atom than zinc. A reducing agent is a substance that loses or gives up electrons to another substance in a redox reaction. +3 0 Reducing Agent + Fe2O3(s) → Fe + other products ↓ ↑ ↓ _______↑ reduction An oxidizing agent is a substance that gains or removes electrons from another substance in a redox reaction _______ │ ↓ 0 ↓ Oxidizing agent + MgO → Mg2+ + other products Development of a Redox Table If a positively charged metal ion reacts, then it is usually converted to a metal atom. According to redox theory, this requires a gain of electrons and hence the metal ion is behaving as an oxidizing agent. Similarly, if a metal atom reacts, then it is always converted to a positively charged ion by losing electrons. Metals always behave as reducing agents In all redox reactions, electrons are transferred from a reducing agent to an oxidizing agent Organization of Redox Table By convention, half-reaction equations are written as reductions (i.e., with the electrons on the left). The strongest oxidizing agent (SOA) is at the top left in a table of relative strengths of oxidizing and reducing agents and the strongest reduction agent (SRA) is at the bottom right of the table. The double arrows may indicate an equilibrium in some situations, but here they simply indicate that the half-reactions may be read from left to right (top arrow) or from right to left (bottom arrow) Table1: - Relative Strengths of Oxidizing and Reducing Agents SOA Decreasing Reactivity of Oxidizing Agents ↓ │ ↓ OA- + N e- <═> RA Ag+(aq) + e- < ═> Ag(s) Cu2+(aq) + 2e- <═> Cu(s) ↑ Decreasing reactivity Pb 2+(aq) + 2e- <═> Pb(s) ↑ of reducing agents Zn2+(aq) + 2e- <═> Zn(s) SRA 1 THE SPONTANEITY RULE Redox Spontaneity Rule states that a spontaneous redox reaction occurs only if the oxidizing agent (OA) is above the reducing agent (RA) in a table of relative strengths of oxidizing and reducing agents. OA RA nonspontaneous + spontaneous + ----------------------→ --------------------→ reaction reaction RA OA 9.4: TECHNOLOGY OF CELL AND BATTERIES Cells and Batteries Electric cell is a device that continuously converts chemical energy into electrical energy Battery is a group of two or more electric cells connected in series. Basic Cell design and Properties Each electric cell is composed of two electrodes and one electrolyte. The electrodes are usually two metals or graphite and a metal. The cathode is the positive electrode and the anode is the negative electrode. In an electric circuit, electrons move from the anode through some conducting materials to the cathode. A voltmeter is a device that can be used to measure the energy difference, per unit electric charge, between two points in an electric circuit. The energy difference per unit charge is called the electric potential difference or voltage. The voltage of a cell depends mainly on the chemical composition of the reactants in the cell. Electric current is a measure of the rate of flow of charge past a point in an electric circuit. Electric current is measured by a device called an ammeter in amperes (A). The charge transferred by a cell or a battery is measured in coulomb (C) The power of a cell or battery is the rate at which it produces electrical energy. Power is measured in watt (W), and calculated as the product of the current and the voltage of the battery Laboratory Investigation: 9.4.1 p. 688 Make Notes on Consumer, commercial and Industrial cells p. 688 - 693 9.5: THE GALVANIC CELLS A galvanic cell is an arrangement of two half-cells that can produce electricity spontaneously. An Example of Galvanic Cell: Put Diagram here please: (i) Cu(s) → Cu2+(aq) + 2e- (ii) 2Ag+ + 2e- → 2Ag(s) → 2Ag(s) +Cu(NO3)2(aq) (Oxidation) The overall cell reaction is Cu(s) + 2AgNO3(aq) 2 (Reduction) Reducing Potential Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s) Ag(s) + Cu2+(aq) → NO REACTION Silver ions can remove (oxidize) electrons from Copper atoms but Copper ions cannot oxidize Silver atoms. Copper atoms can give (reduce) electrons to Silver ions but Silver atoms cannot reduce Copper atoms. Copper is a better REDUCING AGENT than silver. Silver is a better OXIDIZING AGENT than Copper The tendency to become reduced = REDUCTION POTENTIAL, (symbol E) Each half-cell has a different Reduction Potential (or Standard Reduction potential on the reduction table] when the Temperature is 25o, Concentration is 1M and; Pressure is 1 atmospheric. When two half-cells are connected, the one with the larger Reduction Potential acquires electrons from the half-cell with lower Eo. SHORTHAND WAY Cu(s)/Cu2+(aq)//Ag+(aq)/Ag(s) For a galvanic cell to work, the solutions in both half-cells must remain electrically neutral. This requires that ions be permitted to enter or leave the solution. A SALT BRIDGE is a tube filled with electrolyte solution commonly KNO 3 or KCl and fitted with porous plug at either end. The function of a salt bridge is to maintain electrically neutrality in both half-cells. The Galvanic cell has an ability to push electrons through the external circuit. This is called the CELL POTENTIAL. E cell measured in Volts (V). The standard Cell potential E o cell Concentration = IM T = 25oC P = 1 Atm. To calculate the Standard cell potential for the Galvanic Cell is: Eo Cell = EorCathode - Eor Anode Note: For the Reaction to be spontaneous Eo Cell > 0 Example: Draw an electrochemical cell diagram with the following composition Mg(s)/Mg2+(aq)//Fe2+(aq)/Fe(s) Label and identify the Anode, Cathode, Oxidizing agent, Reducing Agent, Salt-bridge and flow of electrons. Write equations for both half-cells and calculate the overall cell potential. Anode: - The electrical conductor at which oxidation occurs. The electrons released by the oxidation flow from the Anode to the external circuit. Cathode: - The electrical conductor at which reduction occurs. 3 Circuit: - Is the external path that electrons take or flow from one electrode through a conductor back to the other electrode. Cell Potential (EoCell) Positive: - Indicates that the reaction tends to proceed spontaneously Negative: - The reverse reaction tends to proceed. (It does not mean that no reaction) A net equation for an Oxidation-Reduction reaction can be obtained by combining any two half-cell reactions in the Table, so that electrons algebraically cancel. One half-cell is undergoing reduction. (The one with high Reduction Potential), and the other half-cell will be oxidized. Example: - Cu(s)/Cu2+(1M)//Ag (1M)/Ag(s) Ag+ + ē ↔ Ag(s) Eo = 0.80V (Cathode electrode (Reduction) Cu2+ + 2 ē ↔ Cu(s) Eo = 0.34V (Anode Electrode Oxidation) (i) Ag+ ē ↔ Ag(s) Eo = 0.80V ↔ Cu2+ +2ē + (ii) Cu(s) 2Ag+ + 2ē +Cu(s) ↔ 2Ag(s) Eo Cell = Eo = -0.34V + Cu2+ + 2ē 0.46V Example: - Calculate the voltage for the following electrochemical cells. (a) Mg2+ + 2ē ↔ Mg(s) with Fe2+ + 2 ē → Fe(s) Measuring Cell Potential It is possible to predict whether a given oxidation reaction will actually occur by using ReductionPotential Table (Ability to be reduced or ability to take electrons) These potentials are obtained by measuring the voltages of electrochemical cells, each of which is made up of two half-cells, by placing a voltmeter across the two terminals For example1: Zn/Zn2+ // Ni2+/Ni – Connected by a salt bridge will indicate that electrons tend to flow from Zn half-cell to Ni half-cell and the potential difference is 0.5 V. Example 2: Ag+/Ag(s) // Zn / Zn2+ - Electrons will flow from Zn to Ag with the potential difference Eocell of 1.5 Volts. Therefore Ag+/Ag has 1.5 Volts more electron attracting ability than Zn/Zn2+ Example 3: H2/2H+ - It’s used as a standard half cell with the potential of Zero If you connect: Ag / Ag+ // 2H+ / H2 – Eocell is 0.80 V Zn / Zn+ // 2H+ / H2 – Eocell is 0.76 V (the ability of Zn2+ to be reduced is 0.76 V less than that of H+) o SOA E Ag+ + e- → Ag(s) 0.80 V I2 + 2e- → 2I0.54 V 4 1. 2. 3. 2H+ + 2e- → H2 0.00 V Zn2+ + 2e- → Zn(s) - 0.76 V 2H2O + 2e- → H2 + 2OH- -0.83 V Na+ + e- → Na(s) - 2.71 V SRA The standard potential difference between two half-cells in their standard state is the measurement of the driving force of the reaction at 25o C, pressure 1 Atm. and Concentration of 1M. SOA half-cell will be reduced by gaining electrons (CATHODE) SRA half-cell will be oxidized by losing electrons (ANODE) Eocell > 0 indicates that the reaction tends to proceed spontaneously Eocell < 0 indicates reverse reaction tends to proceed. It does not mean that there is no reaction. TEST QUESTIONS Balance the following equation by half- reaction method. MnO4+ Fe2+ + H+ → Mn2+ + Fe3+ Balance the following reaction using the oxidation number method. HIO3 + H2SO3 → I2 + H2SO4 + H20 Use the half-reaction method to balance the following equation in basic conditions. S2O32- + NiO2 → Ni(OH)2 + SO32- TEST QUESTIONS 1. Balance the following equation using the half-cell method in acidic condition. NO + Sn → NH2OH + Sn2+ 2. Use the oxidation number method to balance the following equation. Cl+ CrO42- → ClO+ CrO2- 3. Use the oxidation number method to balance the following equation PbSO4 → Pb + PbO2 + SO42- 5









