Bonding Worksheet
advertisement
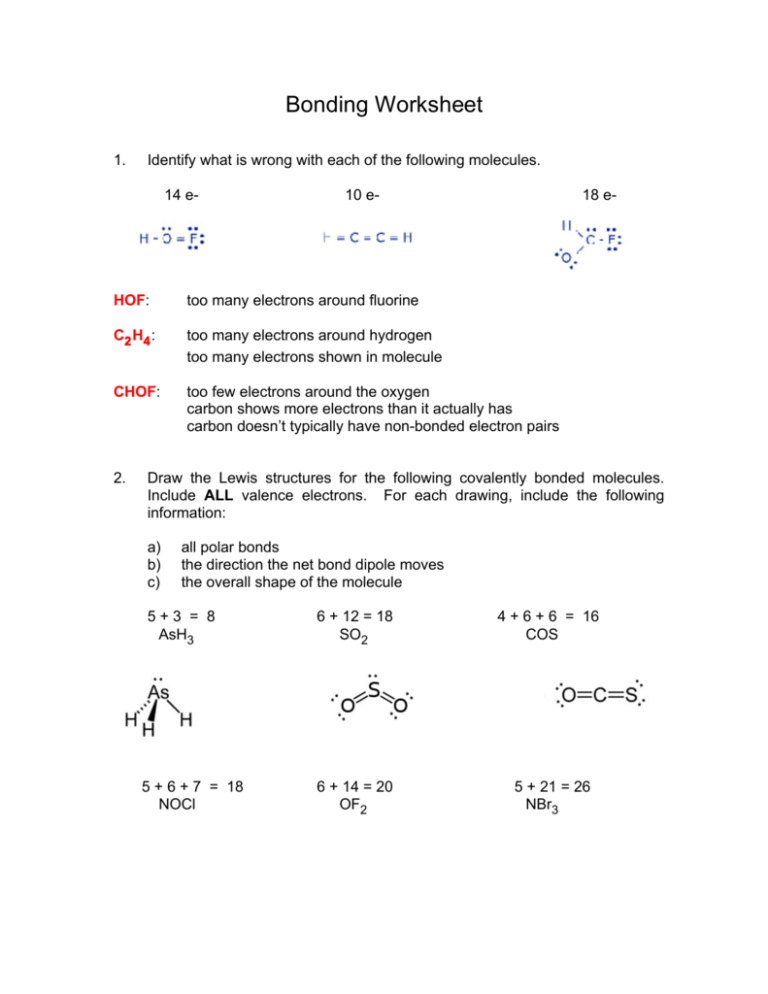
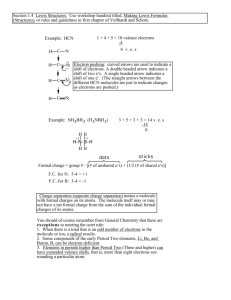
Bonding Worksheet 1. Identify what is wrong with each of the following molecules. 14 e- 10 e- 18 e- HOF: too many electrons around fluorine C2 H4 : too many electrons around hydrogen too many electrons shown in molecule CHOF: too few electrons around the oxygen carbon shows more electrons than it actually has carbon doesn’t typically have non-bonded electron pairs 2. Draw the Lewis structures for the following covalently bonded molecules. Include ALL valence electrons. For each drawing, include the following information: a) b) c) all polar bonds the direction the net bond dipole moves the overall shape of the molecule 5+3 = 8 AsH3 5 + 6 + 7 = 18 NOCl 6 + 12 = 18 SO2 4 + 6 + 6 = 16 COS 6 + 14 = 20 OF2 5 + 21 = 26 NBr3 3. Work on questions 8.97, 98, 99, 100. Answers are found online on my website. 4. Three Lewis structures representing HONO2 is shown below. A a) C Identify the formal charges on each atom in each drawing. A O +1 N +1 N - O - -1 b) B B O N O=N O-N 0 0 0 -1 C O N O=N 0 0 0 Based on these formal charges, which is the best Lewis structure? C is the best drawing since all formal charges are zero, representing the lowest potential energy possible.