WS 7 - Oregon State University
advertisement

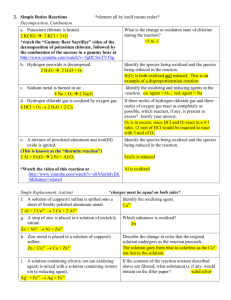
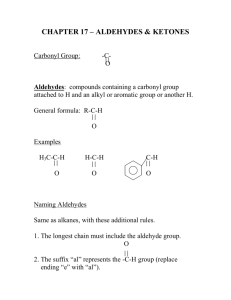
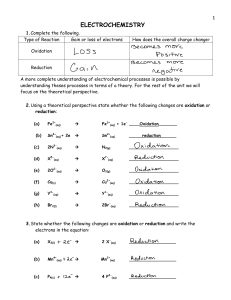
Chemistry 130 Worksheet 7 Oregon State University Dr. Richard Nafshun 1. Draw an aldehyde that contains six carbon atoms. Identify the carbonyl group (C=O) portion in the molecule. 2. Draw a ketone that contains six carbon atoms. Identify the carbonyl group (C=O) portion in the molecule. 3. What is the difference between an aldehyde and a ketone? 4. The molecule shown below is: a. b. c. d. e. an aldehyde. a ketone. a carboxylic acid. an amine. all of the above. 5. The molecule shown below is: a. b. c. d. e. an aldehyde. a ketone. a carboxylic acid. an amine. all of the above. 6. Consider: Cu2+ (s) + H2 (g) → Cu (s) + 2 H+ (aq) i. Which species is gaining electrons? __________ ii. How do you know that electrons are being transferred? iii. Which species is being reduced? ______________ iv. Which species is being oxidized? ______________ v. Which species is the reducing agent? ______________ vi. Which species is the oxidizing agent? ______________ 7. Predict the outcome of the following reactions (if no reaction occurs, explain why): a) b) c) 8. Starting with methane, show the individual oxidation reactions leading to carbon dioxide. 9. Starting with 2-methylbutane, show the individual oxidation reactions leading to the carboxylic acid. [Note: depending on which carbon you choose to oxidize in the alkane, different products can be formed]. 10. What is meant by "redox?" 11. What is meant by "a species is oxidized?" 12. What is meant by "a species is reduced?" 13. What is meant by "a species is an oxidizing agent or oxidant?" 14. What is meant by "a species is a reducing agent or reductant?" 15. Consider the reaction Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s). Which of the following statements is false? a. Zn (s) is being oxidized. b. c. d. e. Cu2+ (aq) is being reduced. Cu2+ (aq) is gaining electrons. Zn2+ (aq) is the oxidizing agent. Two moles of electrons are transferred for each mole of Cu (s) generated. 16. Consider: 2 Al0 (s) + 6 H+ (aq) → 2 Al3+ (aq) + 3 H20 (g). i. Which species is gaining electrons? ______________ ii. How do you know that electrons are being transferred? iii. Which species is being reduced? ______________ iv. Which species is being oxidized? ______________ v. Which species is the reducing agent? ______________ vi. Which species is the oxidizing agent? ______________ 17. Consider the following unbalanced redox equation which takes place in acid: MnO4- (aq) + C2H2O4 (aq) ---> Mn2+ (aq) + CO2 (g) Looking at the reaction above, i. Which species is being reduced? ______________ ii. Which species is being oxidized? ______________ 18. Consider the following redox equation: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O i. Which species is being reduced? ii. Which species is being oxidized? iii. What biological process does this reaction represent? 19. Are redox reactions occurring in you, right now?