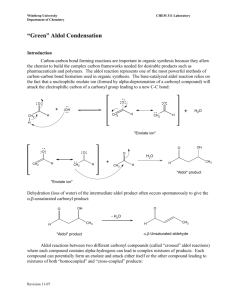
Reactions at the a-carbon in carbonyl compounds
advertisement
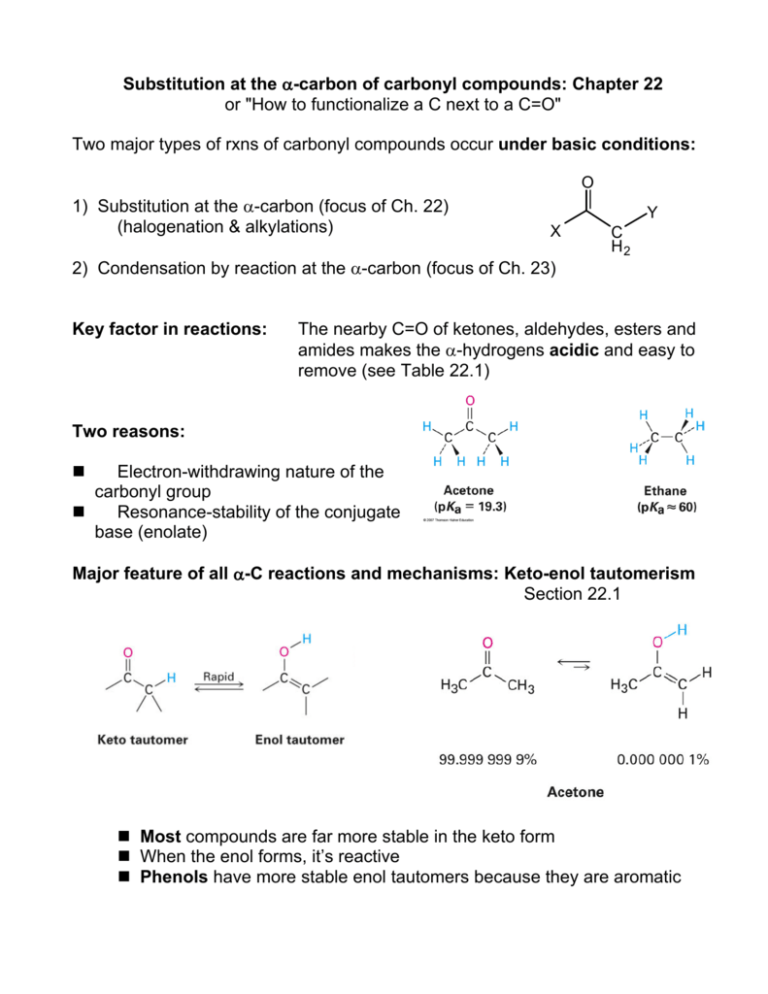
Substitution at the α-carbon of carbonyl compounds: Chapter 22 or "How to functionalize a C next to a C=O" Two major types of rxns of carbonyl compounds occur under basic conditions: O 1) Substitution at the α-carbon (focus of Ch. 22) (halogenation & alkylations) Y X 2) Condensation by reaction at the α-carbon (focus of Ch. 23) Key factor in reactions: C H2 The nearby C=O of ketones, aldehydes, esters and amides makes the α-hydrogens acidic and easy to remove (see Table 22.1) Two reasons: Electron-withdrawing nature of the carbonyl group Resonance-stability of the conjugate base (enolate) Major feature of all α-C reactions and mechanisms: Keto-enol tautomerism Section 22.1 Most compounds are far more stable in the keto form When the enol forms, it’s reactive Phenols have more stable enol tautomers because they are aromatic Mechanism of acid-catalyzed substitution (enol form): H H O H+ R O R R R H E+ O O R R R R H H E AH H O O O R R R H R R R E H E Key acid-catalyzed alpha-C substitution reactions: A. Acid-catalyzed alpha-halogenation (22.3) Br2, Cl2 or I2 can substitute at the α-carbon of an aldehyde or ketone O O Br 2 H 3C C H2 CH3 H 3C HOAc CH CH3 Br Main uses: 1. Allows the α-carbon to be functionalized by SN2 substitutions 2. Provides route to α, β unsaturated ketones by elimination B. Hell-Volhard-Zelinski (HVZ) reaction: Carboxylic acids normally don’t enolize, so this reaction forms an acyl bromide that does enolize and then undergoes α-bromination. Hydrolysis gives back the carboxylic acid: O O 1. Br2, PBr 3 H 3C C H2 OH 2. H2O H 3C CH Br OH Enolates In the presence of strong base, an α-hydrogen can be removed to create a carbanion that is resonance-stabilized through formation of an enolate species: O R R O O -:B R R H H R R H H Two nucleophilic sites are produced : the α-carbon and the oxygen. Some strong bases: Lithium diisopropyl amide (LDA) Sodium ethoxide (Na+ -OEt) NaOH, NaNH2 Formation of an enolate by LDA: In a based-catalyzed α-substitution: • The nucleophile is a carbanion generated by deprotonation at α-C • The electrophile can be varied for each reaction, to give variety of products. Substitution reactions involving the enolate intermediate result in replacement of acidic H by halogen or alkyl group A) Halogenations: 22.6 B) Alkylations: 22.7 Iodoform reaction (base-catalyzed) Direct α-alkylation of ketones, esters & nitriles Malonic Ester Synthesis of substituted esters or carboxylic acids Acetoacetic Acid synthesis of methyl ketones A) Base-catalyzed halogenation: Iodoform reaction: Classification test to identify a methyl ketone O O CH3 H3C O -OH I2, NaOH CI 3 H3C O H3C + CHI 3 B) Alkylation: Base-catalyzed substitution of alkyl groups at the α-position B1) Strong base (LDA) deprotonates the α-carbon of a ketone, ester or nitrile. Enolate species is a good nucleophile, undergoes SN2 reaction with alkyl halides: O H3 C C C H2 CH 3 O O LDA, THF CH 3CH2 I C H 3C CH 3 C H H 3C O C H 3C C H Steric hindrance can determine what the major product will be H3 C CH 3 C H2 C CH CH 3 B2) Base-catalyzed diester alkylation: Activation of the “sandwiched” α-carbon of diethyl malonate A practical example: Synthesis of active barbiturates O Barbituric acid and its active derivatives are heterocyclic rings that can be synthesized in 2 parts by condensation. The bottom half HN comes from a diester, diethyl malonate; the top half from urea NH O O R R' The substituted barbiturates have sedative, hypnotic and anaesthetic properties that vary with the chain length and structure of R groups Amobarbital R = ethyl R’ = isoamyl Pentobarbital Phenobarbital R = ethyl R = ethyl R = 2-pentyl R = phenyl Part 1 of the synthesis is α-alkylation of diethyl malonate: O O C C C H2 EtO NaOEt or K2 CO 3 O O C C EtO OEt C H O O Br(CH 2) 2CH(CH 3) 2 EtO OEt C C OEt CH H 2C CH C H2 Part 2: Repeat with bromoethane to put the ethyl group on Part 3: Condensation reaction with urea and strong base to complete ring O O H 2N NH 2 + C Et HN NH C C EtO NaOEt EtOH O O OEt C 5H 11 O O Et C 5H 11 CH 3 CH3 B3) Malonic ester synthesis: Base-catalyzed alkylation followed by hydrolysis and decarboxylation is used to prepare longer carboxylic acids from alkyl halides Overall reaction: CH2(CO2Et)2 1. Base 2. R-X 3. H3O+ RCH2COOH + CO2 + EtOH Example: How can you prepare these using malonic ester synthesis? B4) Acetoacetic ester synthesis is used to prepare methyl ketones from alkyl halides Using acetoacetic acid synthesis to prepare 2-pentanone: How would you prepare: How could you prepare the substituted ester shown? Show the step-by-step mechanism, including resonance forms, and the final product(s) of this base-catalyzed alkylation reaction: O H3C H3C CH3 C C CH3 H2 LDA/THF CH3CH2I Fill in the reagents needed to accomplish the transformation shown: Reactions at the alpha-carbon, Part II: Additions and condensations (Chapter 23) 1. Common and biologically relevant additions/condensations: Formation of new C – C bonds with loss of water A) Aldol Reactions: Aldol Addition 23.1 (preparation of β-hydroxy aldehydes or ketones) Aldol Condensation to form enones (α, β unsaturated ketones) Mixed Aldol Intramolecular (cyclic) aldol (yields primarily 5 or 6 membered rings) 23.3 – 23.4 23.5 23.6 B) Claisen Reactions: Claisen Condensation & Mixed Claisen Condensation (preparation of β-keto esters or β-diketones) 23.7 - 23.8 Dieckmann cyclization (forms cyclic β-keto esters) 23.9 2. Special addition & elimination reactions with synthetic utility A) Michael Addition: Conjugate addition of enolates to 23.10 α,β-unsaturated carbonyls ->1,5-dicarbonyls B) Stork Enamine reaction: Conjugate addition of enamines to 23.11 α,β-unsaturated carbonyls followed by hydrolysis (forms 1,5-diketones) 1. Additions and condensations between aldehydes, ketones & esters Review concepts: • Carbonyl compounds have acidic H at the α-position • Deprotonation at this position produces a resonance-stabilized carbanion/enolate • This reacts readily with electrophilic site of another molecule Synthesis considerations: • Focus on the functional groups that form in each reaction • Keep track of which reagents supply which carbons and how they connect • Condensations only require a catalytic amount of base 1A: Aldol addition: produces β-hydroxy aldehydes & ketones (“aldols”): O 2 NaOH C H 3C O OH H H3C C H C H C H2 Mechanism: The ensuing condensation (dehydration) of the aldol produces α, βunsaturated ketones Example: The mixed aldol condensation of benzaldehyde and acetone • Mixed aldols best when one reagent has no α-carbons • One serves as nucleophile, the other as electrophile • The dehydration step produces α,β-unsaturated product O O CH O NaOH C + H 3C CH3 heat or acid C H H C + H2O C CH3 1B: Claisen condensation: β-keto esters and β-diketones Ester + ester produces β-keto esters (precursor of acetoacetic acid synthesis): O O 2 H 3C C O H2 C 1. NaOCH2CH3 CH3 2. H3O + O C C H3C C H2 H2 O C CH3 Ketone + ester produces β-diketones: O + C H 3C CH3 O 1. NaOCH3 C 2. H3O + O CH3 H3C O O C C C H2 + CH3OH The main difference between the aldol and Claisen reactions: Claisen reaction involves elimination of a leaving group, regenerating C=O! Intramolecular condensations: Treating dicarbonyl compounds with base can promote cyclizations by aldol or Claisen. Products form in a way that maximizes ring stability (favoring 5 or 6 membered rings). Some examples: Dieckmann cyclizations Claisen, works well with 1,6- or 1,7-diesters: Aldol & Claisen reactions are very common in nature! Some biological examples: A. Aldol addition of two 3-carbon units B. Cross-linking of collagen protein to make a 6-carbon unit occurs during as animals age: an aldol condensation: gluconeogenesis: C. Claisen condensation of thioesters (malonyl-CoA and acetyl-CoA) occurs in fatty acid chain-building (biosynthesis) 2. Special Additions/Eliminations 2A. Michael Additions of α, β−unsaturated carbonyl compounds Recall that there are 2 positively charged sites in these species; they can undergo both direct addition and conjugate addition: In the Michael reaction, the nucleophile is an enolate species and conjugate addition to the unsaturated structure produces a multifunctionalized carbonyl compound: The products have the new group attached at the β-carbon The enolate can come from a β-diketone, β-diester or β-keto ester When the reactant is an ester, the base must have the same alkyl group to avoid any change in the molecule if substitution occurs. The resulting 1,5-diketone can undergo a Robinson annulation 2B. Stork Reaction: Addition of enamines to α, β-unsaturated carbonyls to produce 1,5-diketones Ketones can be converted to enamines and used to alkylate α,β-unsaturated carbonyl compounds because enamines have a carbanion resonance form: R2 N R2 N The 3-step process results in formation of 1,5-diketones (which are synthetically useful in cyclizations): 1. Enamine formation by reaction of ketone with a 2o amine 2. Michael addition of enamine to α, β-unsaturated carbonyl compound 3. Hydrolysis of the enamine regenerates the ketone group. 1,5-diketones prepared in this way may undergo intramolecular aldol condensation (annulation) forming a new 6-membered ring O O OH NaOH heat O O Problems: What enone product would form from aldol condensation in each of these molecules? What aldol condensation product would form from treatment of this compound with base? How might each compound be prepared using a Michael reaction? Show which nucleophilic donor and electrophilic acceptor you would use (Table 23.1) Fill in the missing reagents Predict the products formed from a Michael addition, followed by intramolecular aldol condensation (Robinson annulation): 1) 2,4-pentanedione + 2-cyclohexenone 2)