Chapter 22- important concept
advertisement

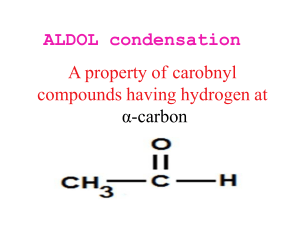
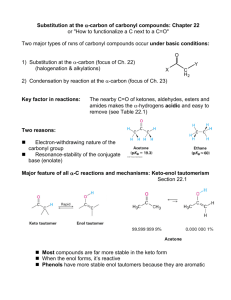
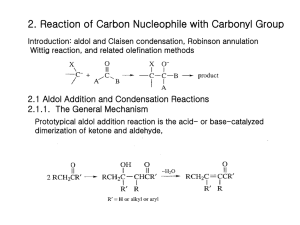
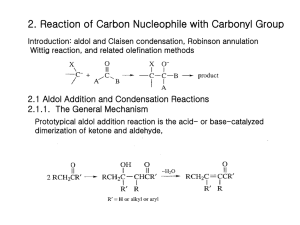
Chapter 22- important concept. Reactions: you should know the following reactions, and also be able to draw the mechanism (acid and/or base catalyzed): - halogenation haloform reaction HVZ reaction alkylation of ketones (via LDA, or by enamine formation) aldol condensation of ketones and aldehydes, followed by dehydration crossed aldol condensation (pay attention to the best strategy to minimize unwanted products)) Claisen condensation of esters, crossed Claisen and intramolecular (Dieckmann) Malonic ester synthesis of substituted acids Acetoacetic ester synthesis of substituted ketones Michael addition (enolate to unsaturated ketones) Robinson annulation Things to remember: the first step in an acid catalyzed mechanism is always the protonation of the carbonyl, and in a base catalyzed reaction it’s the abstraction of a proton to form an enolate. Acidity of protons: ketones (pKa~20) are more acidic than eters (pKa~24), 1,3 dicarbonyl compounds are more acidic (pKa~10), with diketones > ketoesters > diesters. Also, try to focus on the products formed in each reaction, and to the C-C bonds formed. For example, substituted ketones can be made by direct alkylation of a ketone (using LDA as base, or activating the carbonyl via enamine formation) with an alkyl halide (SN2 mechanism), or by acetoacetic ester synthesis (alkylation of a 1,3 (or ) ketoester followed by acid hydrolysis (heat)); substituted acids can be made by malonic ester synthesis (alkylation of a 1,3 (or ) diester followed by acid hydrolysis (heat)); 1,5 (or ) diketones and ketoesters can be made by Michael addition of an enolate, typically a 1,3 (or dicarbonyl compound, to a unsaturated ketone or aldehyde; in turn, unsaturated ketones or aldehydes can be made by aldol condensation.