E-Aldol-Condensation
advertisement
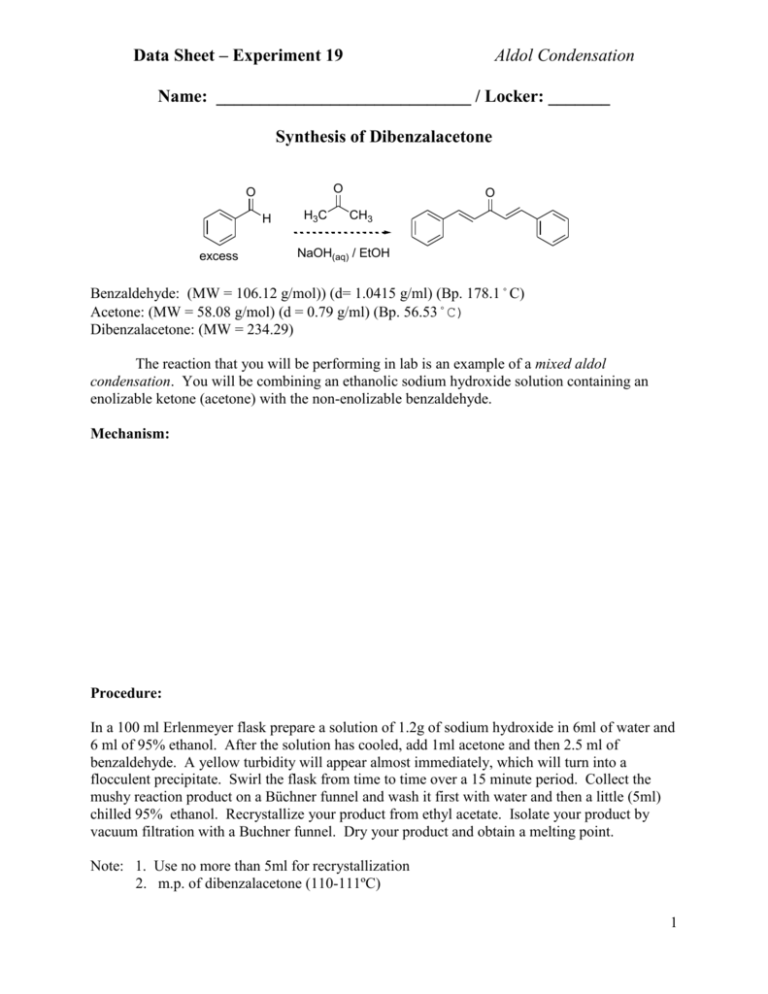
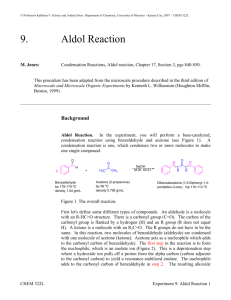
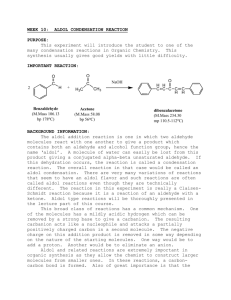
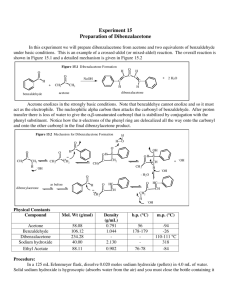
Data Sheet – Experiment 19 Aldol Condensation Name: _____________________________ / Locker: _______ Synthesis of Dibenzalacetone O O H excess H3C O CH3 NaOH(aq) / EtOH Benzaldehyde: (MW = 106.12 g/mol)) (d= 1.0415 g/ml) (Bp. 178.1˚C) Acetone: (MW = 58.08 g/mol) (d = 0.79 g/ml) (Bp. 56.53˚C) Dibenzalacetone: (MW = 234.29) The reaction that you will be performing in lab is an example of a mixed aldol condensation. You will be combining an ethanolic sodium hydroxide solution containing an enolizable ketone (acetone) with the non-enolizable benzaldehyde. Mechanism: Procedure: In a 100 ml Erlenmeyer flask prepare a solution of 1.2g of sodium hydroxide in 6ml of water and 6 ml of 95% ethanol. After the solution has cooled, add 1ml acetone and then 2.5 ml of benzaldehyde. A yellow turbidity will appear almost immediately, which will turn into a flocculent precipitate. Swirl the flask from time to time over a 15 minute period. Collect the mushy reaction product on a Büchner funnel and wash it first with water and then a little (5ml) chilled 95% ethanol. Recrystallize your product from ethyl acetate. Isolate your product by vacuum filtration with a Buchner funnel. Dry your product and obtain a melting point. Note: 1. Use no more than 5ml for recrystallization 2. m.p. of dibenzalacetone (110-111ºC) 1 Data Sheet – Experiment 6 Aldol Condensation Melting point of your dibenzalacetone: _________________ºC Actual yield: ___________ grams Theoretical yield calculation: Theoretical yield: __________ grams Percent yield calculation: Percent yield: __________ % Discussion: 2