DAT General Chemistry – Problem Drill 20: Lewis Structures
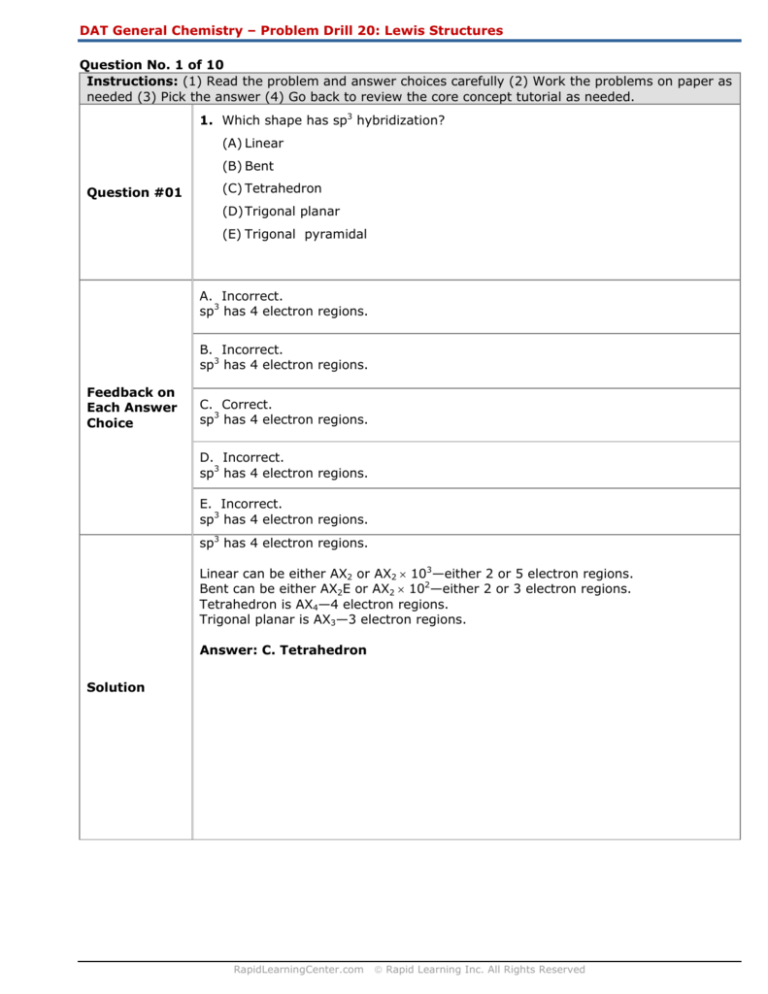
Question No. 1 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
1. Which shape has sp3 hybridization?
(A) Linear
(B) Bent
Question #01
(C) Tetrahedron
(D) Trigonal planar
(E) Trigonal pyramidal
A. Incorrect.
sp3 has 4 electron regions.
B. Incorrect.
sp3 has 4 electron regions.
Feedback on
Each Answer
Choice
C. Correct.
sp3 has 4 electron regions.
D. Incorrect.
sp3 has 4 electron regions.
E. Incorrect.
sp3 has 4 electron regions.
sp3 has 4 electron regions.
Linear can be either AX2 or AX2 × 103—either 2 or 5 electron regions.
Bent can be either AX2E or AX2 × 102—either 2 or 3 electron regions.
Tetrahedron is AX4—4 electron regions.
Trigonal planar is AX3—3 electron regions.
Answer: C. Tetrahedron
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 2 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
2. Which best describes HCOOH?
Unshared
electron pairs
Question #02
Shared
electron pairs
in a single
bond
2
1
2
3
2
Shared
electron pairs
in a double
bond
2
2
1
2
2
A
0
B
0
C
1
D
4
E
2
A. Incorrect.
First draw the Lewis structure of HCOOH, then start the counting. There are 3
bonded pairs in single bonds, 2 bonded pairs in a double bond and 4 unshared
electron pairs.
B. Incorrect.
First draw the Lewis structure of HCOOH, then start the counting. There are 3
bonded pairs in single bonds, 2 bonded pairs in a double bond and 4 unshared
electron pairs.
Feedback on
Each Answer
Choice
C. Incorrect.
First draw the Lewis structure of HCOOH, then start the counting. There are 3
bonded pairs in single bonds, 2 bonded pairs in a double bond and 4 unshared
electron pairs.
D. Correct.
First draw the Lewis structure of HCOOH, then start the counting. There are 3
bonded pairs in single bonds, 2 bonded pairs in a double bond and 4 unshared
electron pairs.
E. Incorrect.
First draw the Lewis structure of HCOOH, then start the counting. There are 3
bonded pairs in single bonds, 2 bonded pairs in a double bond and 4 unshared
electron pairs.
Draw the Lewis structure for HCOOH.
HCOOH is a carboxylic acid with a hydrogen on the other side of the carbon in the
carboxylic acid.
Solution
There are 3 bonded pairs in single bonds, 2 bonded pairs in a double bond and 4
unshared electron pairs.
Answer: D
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 3 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
3. Which gives the correct comparison of the carbon-carbon bond characteristics in
C2H4 and C2H2? Compared to C2H4, the properties for C2H2 are:
Question #03
A
B
C
D
E
Bond length
Bond strength
Greater
Greater
Smaller
Smaller
Similar
Smaller
Smaller
Smaller
Greater
Similar
Number of
shared electron
pairs
Greater
Smaller
Smaller
Greater
Same
A. Incorrect.
C2H4 has a carbon-carbon double bond while C2H2 has a carbon-carbon triple bond.
Draw the Lewis structures for both. The greater the bond strength and the smaller
in bond length.
B. Incorrect.
C2H4 has a carbon-carbon double bond while C2H2 has a carbon-carbon triple bond.
Draw the Lewis structures for both. The greater the bond strength and the smaller
in bond length.
Feedback on
Each Answer
Choice
C. Incorrect.
C2H4 has a carbon-carbon double bond while C2H2 has a carbon-carbon triple bond.
Draw the Lewis structures for both. The greater the bond strength and the smaller
in bond length.
D. Correct.
C2H4 has a carbon-carbon double bond while C2H2 has a carbon-carbon triple bond.
Draw the Lewis structures for both. The greater the bond strength and the smaller
in bond length.
E. Incorrect.
C2H4 has a carbon-carbon double bond while C2H2 has a carbon-carbon triple bond.
Draw the Lewis structures for both. The greater the bond strength and the smaller
in bond length.
Draw the structures for each molecule. Each increase in shared electrons results in
stronger and shorter bonds.
Solution
C2H2 has a triple C-C bond. Therefore, it has a smaller bond length, a higher bond
strength and a greater number of shared electrons.
Answer: D
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 4 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
4. Give the VSEPR shape for CO3-2
Question #04
A.
B.
C.
D.
E.
Trigonal planar
Trigonal pyramidal
Tetrahedron
Bent
Linear
A. Correct.
Three bonding regions and no lone pairs for the central atom is trigonal planar
geometry.
B. Incorrect.
The central atom of this molecule has 3 bonding regions and no lone pairs.
Determine the geometry accordingly.
Feedback on
Each Answer
Choice
C. Incorrect.
The central atom of this molecule has 3 bonding regions and no lone pairs.
Determine the geometry accordingly.
D. Incorrect.
The central atom of this molecule has 3 bonding regions and no lone pairs.
Determine the geometry accordingly.
E. Incorrect.
The central atom of this molecule has 3 bonding regions and no lone pairs.
Determine the geometry accordingly.
CO3-2 has three bonding regions and no long pair regions. This is trigonal planar
shape.
Answer: A. Trigonal Planar
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 5 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
5. Which has a larger Cl-P-Cl bond angle, PCl3 or PCl4+?
Question #05
(A)
(B)
(C)
(D)
(E)
PCl3
PCl4+
Exactly the same
Cannot be determined
Similar but not the same
A. Incorrect.
PCl3 has a lone pair while the ion does not. Remember lone pairs take more space
than bonding pairs.
B. Correct.
PCl3 has a lone pair while the ion does not. Remember lone pairs take more space
than bonding pairs.
Feedback on
Each Answer
Choice
C. Incorrect.
PCl3 has a lone pair while the ion does not. Remember lone pairs take more space
than bonding pairs.
D. Incorrect.
PCl3 has a lone pair while the ion does not. Remember lone pairs take more space
than bonding pairs.
E. Incorrect.
PCl3 has a lone pair while the ion does not. Remember lone pairs take more space
than bonding pairs.
They both have 4 electron regions. PCl3 has 1 lone pair while PCl4+ has none. Lone
pairs distort bond angles in towards each other. Therefore PCl4+ has a higher bond
angle.
Answer: (B) PCl4+
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 6 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
6. What is the electron geometry for H2S?
Question #06
(A) Linear
(B) Bent
(C) Trigonal Planar
(D) Tetrahedron
(E) Trigonal pyrimidal
A. Incorrect.
There are two bonding regions and two lone pair regions—that’s four electron
regions total.
B. Incorrect.
There are two bonding regions and two lone pair regions—that’s four electron
regions total.
Feedback on
Each Answer
Choice
C. Incorrect.
There are two bonding regions and two lone pair regions—that’s four electron
regions total.
D. Correct.
There are two bonding regions and two lone pair regions—that’s four electron
regions total.
E. Incorrect.
There are two bonding regions and two lone pair regions—that’s four electron
regions total.
Each bond and lone pair count as 1.
4 electron regions
Answer: D. Tetrahedron
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 7 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
7. What is the molecular geometry for H2S?
Question #07
(A)
(B)
(C)
(D)
(E)
Linear
Bent
Trigonal planar
Tetrahedron
Trigonal pyrimidal
A. Incorrect.
There are two bonding regions and two lone pair regions.
B. Correct.
There are two bonding regions and two lone pair regions.
Feedback on
Each Answer
Choice
C. Incorrect.
There are two bonding regions and two lone pair regions.
D. Incorrect.
There are two bonding regions and two lone pair regions.
E. Incorrect.
There are two bonding regions and two lone pair regions.
2 lone pairs, 2 ligands
Answer: B. Bent
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 8 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
8. Which molecular geometry ALWAYS results in polar molecules?
Question #08
(A)
(B)
(C)
(D)
(E)
Tetrahedron
Trigonal bipyramidal
Trigonal pyramidal
Octahedron
Linear
A. Incorrect.
Although all of them can produce polar molecules, one of them has a lone pair of
electrons that always results in a net dipole.
B. Incorrect.
Although all of them can produce polar molecules, one of them has a lone pair of
electrons that always results in a net dipole.
Feedback on
Each Answer
Choice
C. Correct.
Although all of them can produce polar molecules, trigonal planar has a lone pair of
electrons that always results in a net dipole.
D. Incorrect.
Although all of them can produce polar molecules, one of them has a lone pair of
electrons that always results in a net dipole.
E. Incorrect.
Although all of them can produce polar molecules, one of them has a lone pair of
electrons that always results in a net dipole.
Although all of them can produce polar molecules, Trigonal Pyramidal has a lone
pair of electrons which will always result in a net dipole.
Answer: (C) trigonal pyramidal
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 9 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
9. What is the molecular geometry for CO2?
Question #09
(A)
(B)
(C)
(D)
(E)
Linear
Bent
Trigonal pyramidal
Tetrahedron
Trigonal planar
A. Correct.
There are two bonding regions and no lone pair regions.
B. Incorrect.
There are two bonding regions and no lone pair regions.
Feedback on
Each Answer
Choice
C. Incorrect.
There are two bonding regions and no lone pair regions.
D. Incorrect.
There are two bonding regions and no lone pair regions.
E. Incorrect.
There are two bonding regions and no lone pair regions.
Carbon dioxide is a central carbon with 2 bonding regions to the oxygen atoms—
each is a double bond. Double bonds count the same as singles for VSEPR theory.
So it has 2 bonding regions and no lone pair regions.
Answer: (A) Linear
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved
Question No. 10 of 10
Instructions: (1) Read the problem and answer choices carefully (2) Work the problems on paper as
needed (3) Pick the answer (4) Go back to review the core concept tutorial as needed.
10. What is the molecular geometry for PCl5?
Question #10
(A)
(B)
(C)
(D)
(E)
Tetrahedron
Trigonal pyramidal
Trigonal bipyramidal
Octahedron
Bent
A. Incorrect.
There are 5 bonding regions and no lone pair regions.
B. Incorrect.
There are 5 bonding regions and no lone pair regions.
Feedback on
Each Answer
Choice
C. Correct.
There are 5 bonding regions and no lone pair regions.
D. Incorrect.
There are 5 bonding regions and no lone pair regions.
E. Incorrect.
There are 5 bonding regions and no lone pair regions.
PCl5 has 5 bonding regions and no lone pair regions.
Answer: (C) Trigonal bipyramidal
Solution
RapidLearningCenter.com
© Rapid Learning Inc. All Rights Reserved