Notes on the VSEPR Model of Molecular Geometry
advertisement

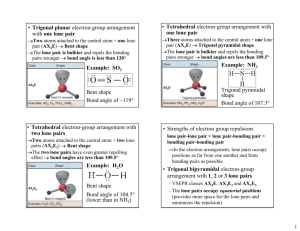
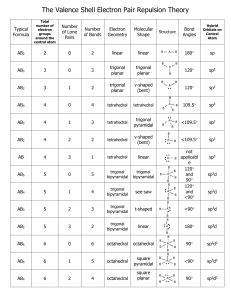
CH101 Notes on the VSEPR Model of Molecular Geometry The valence shell electron pair repulsion (VSEPR) model is a tool of the valence bond method; it is based on Lewis structures and it relates molecular geometry to the atomic hybridization mode of the central atom. Basic Rules for Molecules ABn (A = central atom, B = outer atom) 1. Bonding (single and multiple) electron pairs and nonbonding electron pairs (= “lone pairs”) repel each other and therefore avoid each other, as far as possible, in the geometry of the molecule. 2. The repulsive force of a single bond is less than that of a lone pair or a multiple bond. This is because lone pairs and double bonds occupy more space around the central atom (A). The more space a lone pair occupies, the greater the repulsive effect on bond electrons and the greater the distortion. 3. As the electronegativity difference between the central (A) and outer atoms (B) increases (where B is the more electronegative partner), the BAB bond angle decreases. This is because the bond electrons are localized more and more upon L, hence reducing their repulsive effect on other electron pairs around A. 4. As the size (radius) of A decreases or the size of B increases (where B is the more electronegative partner), the BAB bond angle increases. This is because the electronic and steric repulsions between B increase. Application of rules 1 and 2 to ABnEm (where m is the number of lone pairs of electrons on A) leads to the following arrangements of electron pairs around A (the sum n + m is called the steric number): n+m 2 Idealized Linear shape of (sp) electron pairs around A 3 4 5 Trigonal planar (sp2) Tetrahedral Trigonal (sp3) bipyramidal (sp3d) 6 7 Octahedral Pentagonal (sp3d2) bipyramidal 8 Anticubic The structural names of molecules of the type AB2 through AB6 are given, with examples, in Table 1. Distortion of Bond Angles Note that molecules whose central atom A has lone pairs of electrons or double bonds, as well as single bonds, may possess distorted bond angles, as governed by rule 2. Similarly, the operation of the factors that give rise to rules 3 and 4 may also result in distorted bond angles. This is summarized in Table 2 for some common compounds. 1 Table 1. Structures of Molecules of Type ABn (: = lone pair) n + m Arrangement Type Shape Examples 2 Linear AB2 Linear CO2, HCN, NO2+, BeCl2 (g) 3 Trigonal planar AB3 Trigonal planar :AB2 Bent (V-shape) BCl3, CO32-, COCl2, NO3SO3 O3, SO2, NO2-, SnCl2 (g), PbCl2 AB4 Tetrahedral BF4-, CH4, NH4+, SO42-, SO2Cl2, ClO4-, XeO4, PF3O :AB3 Pyramidal NH3, SOCl2, SO32-, XeO3 ::AB2 Bent (V-shape) AB5 Trigonal bipyramidal :AB4 Pivot or tetrahedron) ::AB3 T-shape ClF3, XeOF2 :::AB2 Linear ICl2-, I3-, XeF2 AB6 Octahedral :AB5 Square-pyramidal ::AB4 Square planar SF6, AlF63-, SiF62-, PF6-, IOF5, XeO2F4 TeCl5-, BrF5, XeOF4, SF5-, SbF52-, ClOF4-, XeF5+ BrF4-, XeF4 4 5 6 Tetrahedral Trigonal bipyramidal Octahedral see-saw H2O, NH2-, SCl2, Cl2O, I3+, XeO2, H2F+ PF5, SbCl5, SOF4, XeO3F2, IO2F3, IO3F2(distorted SbF4-, SeF4, ClOF3, XeO2F2, IO2F2-, SbF4- Table 2. Bond Angles of Tetrahedron-Based Molecules (AB4, :AB3, ::AB2) [increasing in direction shown by arrows] Hydrogen compounds Halogen compounds CH4 (109.5o) NH3 (106.8o) H2O (104.5o) SiH4 (109.5o) PH3 (93.5o) H2S (92.3o) GeH4 (109.5o) AsH3 (92.0o) H2Se (91.0o) SnH4 (109.5o) SbH3 (91.5o) H2Te (89.5o) OF2 (103.7o) OCl2 (111.2o) SF2 (98.2o) SCl2 (102.7o) SeF2 (95.8o) SeCl2 (99.6o) TeF2 (93.3o) TeCl2 (97.0o) NF3 (102.4o) NCl3 (107.4o) PF3 (97.4o) PCl3 (100.1o) AsF3 (96.0o) AsCl3 (98.6o) SbF3 (95.0o) SbCl3 (95.5o) 2 Trigonal Bipyramid- and Octahedron-Based Structures Unlike the idealized tetrahedron or octahedron, in which all the vertices are equivalent, idealized trigonal pyramids have two distinct types of vertices: equatorial (eq) and axial (ax), as shown below. F (ax) F (eq) (eq) F Sb F (eq) F (ax) As a general rule, lone pairs (stronger repellers than single bond pairs) preferentially occupy equatorial positions, because the number of lone pair-bond pair repulsions at 90o is fewer (2, as against 3). The presence of lone pairs has a distorting effect on the bond angles, as illustrated by the examples below. SF4 Pivot or see-saw (distorted tetrahedron) BrF3 Distorted T-shape F(ax) F(eq)) : S F(eq)) F(ax) : F(ax) Br F(eq) F(ax) angle F(ax)S F(ax) = 173o angle F(eq)S F(eq) = 101o : angle F(ax)Br F(eq) = 86o angle F(ax)Br F(ax) = 172o The more symmetrical molecules PF5, PCl5 and SbF5 have regular trigonal bipyramidal shapes. Six electron pairs around the central atom give rise to octahedral, square pyramidal and square planar shapes. The first and last of these are regular (undistorted) provided that the outer atoms are identical (e.g. as in SF6 and XeF4, respectively), whereas the second situation is always distorted: BrF5 (Distorted) square pyramid F F(ax) F Br F F angle F(ax)Br F = 86o (instead of 90o) .. 3


![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)