Valence Shell Electron Pair Repulsion Model & the Shape of
advertisement

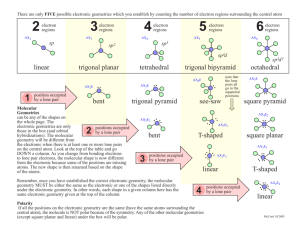
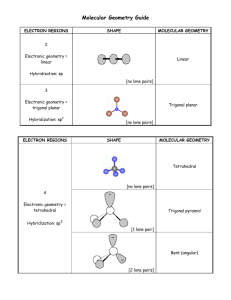
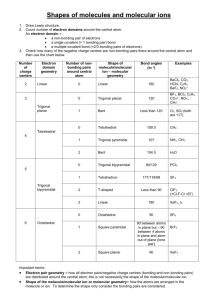
Valence Shell Electron Pair Repulsion Model & the Shape of Molecules For each of the following provide (a) the chemical name, (b) the Lewis structure, (c) the name of the “electron pair” geometry, (d) the name for the “molecular” geometry, (e) a 3D drawing of the compound. 1. CO2 (a) carbon dioxide (b) CO2 has 4+6+6 (=16) valence electrons add 12 O C O O C O more e¯ 4 e¯ (c) C is attached to two atoms and has no lone pairs; therefore the electron pair geometry is linear (d) because C has no lone pairs the molecular geometry is also linear (e) O C O O C O 2. BF3 (a) boron trifluoride (b) BF3 has 3+7+7+7 (=24) valence electrons F B F add 18 F more e¯ F F B F 6 e¯ Note that boron as shown in this Lewis structure violates the octet. Other resonance forms could be written that give boron a complete octet by sharing the electrons in the fluorine lone pair. (c) B is attached to three atoms and has no lone pairs; therefore the electron pair geometry is trigonal planar (d) because B has no lone pairs the molecular geometry is also trigonal planar (e) F F B F F F B F 3. CH4 (a) carbon tetrahydride methane! (b) CH4 has 4+1+1+1+1 (=8) valence electrons H H C H H 8 e¯ done ! H H C H H (c) C is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because C has no lone pairs the molecular geometry is also tetrahedral (e) H H H H C H H H C H 4. PCl5 (a) phosphorus pentafluoride (b) has 5+7+7+7+7+7 (=40) valence electrons Cl Cl P Cl Cl Cl add 30 Cl more e¯ Cl Cl P Cl Cl 10 e¯ (c) P is attached to five atoms and has no lone pairs; therefore the electron pair geometry is trigonal bipyramidal (d) because P has no lone pairs the molecular geometry is also trigonal pyramidal (e) Cl Cl Cl Cl P Cl Cl Cl P Cl Cl Cl 5. SF6 (a) sulfur hexafluoride (b) has 6+7+7+7+7+7+7 (=48) valence electrons F F F S F add 36 F F more e¯ F F F S F F F 12 e¯ (c) S is attached to six atoms and has no lone pairs; therefore the electron pair geometry is octahedral (d) because S has no lone pairs the molecular geometry is also octahedral (e) F F F F S F 6. CCl4 F F F F S F F F (a) carbon tetrachloride (b) has 4+7+7+7+7 (=32) valence electrons Cl Cl C Cl Cl Cl Cl C Cl Cl add 24 more e¯ 8 e¯ (c) C is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because C has no lone pairs the molecular geometry is also tetrahedral (e) Cl Cl Cl C Cl Cl Cl Cl C 7. CH2Cl2 (a) dichloromethane (b) has 4+1+1+7+7 (=20) valence electrons H add 12 H C Cl more e¯ Cl Cl H H C Cl Cl 8 e¯ (c) C is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because C has no lone pairs the molecular geometry is also tetrahedral (e) Cl H H C Cl Cl H H C Cl 8. IF (a) iodine fluoride (b) has 7+7 (=14) valence electrons add 12 I F 2 e¯ I more e¯ (c) has only two atoms, so must be linear (d) linear (doesn’t really have a central atom) (e) I F 9. CS2 (a) carbon disulfide (b) has 4+6+6 (16) valence electrons add 12 S C S more e¯ 4 e¯ I F F S C S (c) C is attached to two atoms and has no lone pairs; therefore the electron pair geometry is linear (d) because C has no lone pairs the molecular geometry is also linear (e) S C S S C S 10. PF6¯ (a) phosphorus hexafluoride (b) has 5+7+7+7+7+7+7+1 (=48) valence electrons F F P F F add 36 F F more e¯ F F F F P F F 12 e¯ (c) P is attached to six atoms and has no lone pairs; therefore the electron pair geometry is octahedral (d) because P has no lone pairs the molecular geometry is also octahedral (e) F F F F P F F F F F F P F F 11. AlCl3 (a) aluminum trichloride (b) has 3+7+7+7 (=24) valence electrons) Cl Cl Al add 18 Cl more e¯ Cl Cl Al Cl 6 e¯ (c) Al is attached to three atoms and has no lone pairs; therefore the electron pair geometry is trigonal planar (d) because Al has no lone pairs the molecular geometry is also trigonal planar (e) Cl Cl Al Cl Cl Cl Al Cl 12. AlCl4¯ (a) aluminum tetrachloride (b) has 3+7+7+7+7+1 Cl Cl Al Cl Cl 8 e¯ add 24 more e¯ Cl Cl Al Cl Cl (c) Al is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because Al has no lone pairs the molecular geometry is also tetrahedral (e) Cl Cl Cl Al Cl Cl Cl Cl Al Cl 13. SiCl4 (a) silicon tetrachloride (b) has 4+7+7+7+7 (=32) valence electrons Cl Cl Si Cl Cl Cl Cl Si Cl Cl add 24 more e¯ 8 e¯ (c) Si is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because Si has no lone pairs the molecular geometry is also tetrahedral (e) Cl Cl Cl Si Cl Cl Cl Cl Si Cl 14. SO3 (a) sulfur trioxide (b) has 6+6+6+6 (=24) valence electrons O O S O add 18 O more e¯ O 6 e¯ S3 O O O S O Note that we make sure to first give oxygen its octet because it is more electronegative. However, the formal charge on each monovalent oxygen (6-1-6 = –1). Since sulfur has no lone pairs its formal charge is 6–3–0 = 3+. Another resonance form results when the oxygen shares its lone pairs with sulfur to give pi bonds and no formal charge. Other resonance structures could be written. (c) S is attached to three atoms and has no lone pairs; therefore the electron pair geometry is trigonal planar (d) because S has no lone pairs the molecular geometry is also trigonal planar (e) O O S 15. H2O (a) dihydrogen monoxide water (b) has 6+1+1 (=8 valence) electrons O O O S O H O H H O H add 4 more e¯ 4 e¯ (c) O is attached to two atoms and has two lone pairs; therefore the electron pair geometry is tetrahedral (d) because O has two lone pairs the molecular geometry is bent (e) H H O O H 16. NH3 (a) nitrogen trihydride ammonia (b) has 5+1+1+1 (=8) valence electrons H H N H H H H N H add 2 more e¯ 6 e¯ (c) N is attached to three atoms and has one lone pair; therefore the electron pair geometry is tetrahedral (d) because N has one lone pair the molecular geometry is trigonal pyramidal (e) H H N H H H N H H H N H trigonal pyramidal tetrahedral 17. NH4+ (a) ammonium (b) has 5+1+1+1+1–1 (=8) valence electrons H H N H done ! H 8 e¯ (c) N is attached to four atoms and has no lone pairs; therefore the electron pair geometry is tetrahedral (d) because N has no lone pairs the molecular geometry is also tetrahedral (e) H H H 18. NO2¯ N H H H H N H (a) nitrite (b) has 5+6+6+1 (=18) valence electrons O N add 14 O O N more e¯ O N O O 4 e¯ Note that if N has two bonds and a lone pair, it has a formal charge of 1+ (5–2–2 ), while monovalent oxygen has a formal charge of 1– (6–1–6). The first resonance structure is nonoctet. Sharing one of the oxygen lone pairs with nitrogen allows it to satisfy the octet rule as shown in the second resonance structure. (c) N is attached to two atoms and has one lone pair; therefore the electron pair geometry is trigonal planar (d) because N has one lone pair the molecular geometry is bent (e) O N O N O O bent trigonal planar 19. O3 (a) ozone (b) has 6+6+6 (=18) valence electrons O O 2 add 14 O O more e¯ O O O O O 4 e¯ Note that if the central O has two bonds and a lone pair, it has a formal charge of 2+ (6–2–2), while monovalent oxygen has a formal charge of 1– (6–1–6). This leaves the oxygen of the first resonance structure without its non-octet. Sharing one of the terminal oxygen lone pairs with nitrogen allows it to satisfy the octet rule as shown in the second resonance structure. (c) the central O is attached to two atoms and has one lone pair; therefore the electron pair geometry is trigonal planar (d) because O has one lone pair the molecular geometry is bent (e) O O O trigonal planar 20. SnCl4 (a) tin tetrachloride (b) has 4+7+7+7+7 (=32) valence electrons O O O bent Cl Cl Sn Cl Cl Cl Cl Sn Cl Cl add 24 more e¯ 8 e¯ (c) Sn is attached to four atoms and no lone pairs; therefore the electron pair geometry is tetrahedral (d) because Sn has no lone pairs the molecular geometry is tetrahedral (e) Cl Cl Cl Cl Sn Cl Cl Cl Sn Cl 21. NO3¯ (a) nitrate anion (b) has 5+6+6+6+1 (=24) valence electrons O O N O add 18 O more e¯ O 6 e¯ N2 O N O O O Note that if N has three bonds and no lone pair (first resonance structure), it has a formal charge of 2+ (5–3), while monovalent oxygen has a formal charge of 1– (6–1–6). The first resonance structure is non-octet. Sharing one of the oxygen lone pairs with nitrogen allows it to satisfy the octet rule as shown in the second resonance structure. (c) N is attached to three atoms and no lone pairs; therefore the electron pair geometry is trigonal planar (d) because N has no lone pairs the molecular geometry is trigonal planar (e) O O N O O O N O 22. SF4 (a) sulfur tetrafluoride (b) has 6+7+7+7+7 (=34) valence electrons F F F S F add 26 F more e¯ F F S F 8 e¯ (c) S is attached to four atoms and one lone pair; therefore the electron pair geometry is trigonal bipyramidal (d) because S has one lone pair the molecular geometry is see-saw (e) F F F F S F S F F F trigonal bipyramidal "see-saw" 23. ClF3 (a) chlorine trifluoride (b) has 7+7+7+7 (=28) valence electrons F Cl add 22 F more e¯ F 6 e¯ F Cl F F (c) Cl is attached to three atoms and two lone pairs; therefore the electron pair geometry is trigonal bipyramidal (d) because Cl has two lone pairs the molecular geometry is T-shaped (e) F F Cl F Cl F F F trigonal bipyramidal "T-shaped" 24. XeF2 (a) xenon diflouride (b) has 8+7+7 (=22) valence electrons F Xe add 18 F more e¯ 4 e¯ F Xe F (c) Xe is attached to two atoms and three lone pairs; therefore the electron pair geometry is trigonal bipyramidal (d) because Xe has three lone pairs the molecular geometry is linear (e) F F Xe Xe F F trigonal bipyramidal 25. BrF5 linear (a) bromine pentafluoride (b) has 7+7+7+7+7+7 (=42) valence electrons F F F Br add 32 F F more e¯ F F F Br F F 10 e¯ (c) Br is attached to five atoms and one lone pair; therefore the electron pair geometry is octahedral (d) because Br has one lone pair the molecular geometry is square pyramidal (e) F F F F Br F F F F F F Br square pyramidal octahedral 26. XeF4 (a) xenon tetrafluoride (b) has 8+7+7+7+7 (=36) valence electrons F F Xe add 32 F F more e¯ F F Xe F F 8 e¯ (c) Xe is attached to four atoms and two lone pairs; therefore the electron pair geometry is octahedral (d) because Xe has two lone pairs the molecular geometry is square planar (e) F F Xe F F F F Xe F F square planar octahedral 27. IF5 (a) iodine pentafluoride (b) has 7+7+7+7+7+7 (=42) valence electrons F F F I 10 e¯ F F add 32 more e¯ F F F I F F (c) I is attached to five atoms and one lone pair; therefore the electron pair geometry is octahedral (d) because I has one lone pair the molecular geometry is square planar (e) F F F F F F F F I F F I square pyramidal octahedral 28. GaH3 (a) gallium trihydride (b) has 3+1+1+1 (=6) valence electrons H H Ga H done ! 6 e¯ Note that this is a violation of the octet. Gallium is in the same group as boron and aluminum, which also form trivalent non-octet structures (see problems #2 and #11). (c) Ga is attached to three atoms and has no lone pairs; therefore the electron pair geometry is trigonal planar (d) because Ga has no lone pairs the molecular geometry is trigonal planar (e) H H Ga H H H Ga H 29. ICl2¯ (a) iodine dichloride (b) has 7+7+7+1 (=22) valence electrons Cl I Cl more e¯ 4 e¯ Cl add 18 I Cl aluminum, which also form trivalent non-octet structures. (c) I is attached to two atoms and has three lone pairs; therefore the electron pair geometry is trigonal planar (d) because I has three lone pairs the molecular geometry is linear (e) Cl Cl I I Cl Cl trigonal bipyramidal linear 30. BrF4¯ (a) bromine tetrafluoride (b) has 7+7+7+7+7+1 (=36) valence electrons F Br F add 28 F more e¯ F F F Br F F 8 e¯ (c) Br is attached to four atoms and has two lone pairs; therefore the electron pair geometry is octaehedral (d) because Br has two lone pairs the molecular geometry is square planar (e) F F Br F F F F Br square planar octahedral F F