Hydrocarbons Lab - faculty at Chemeketa
advertisement
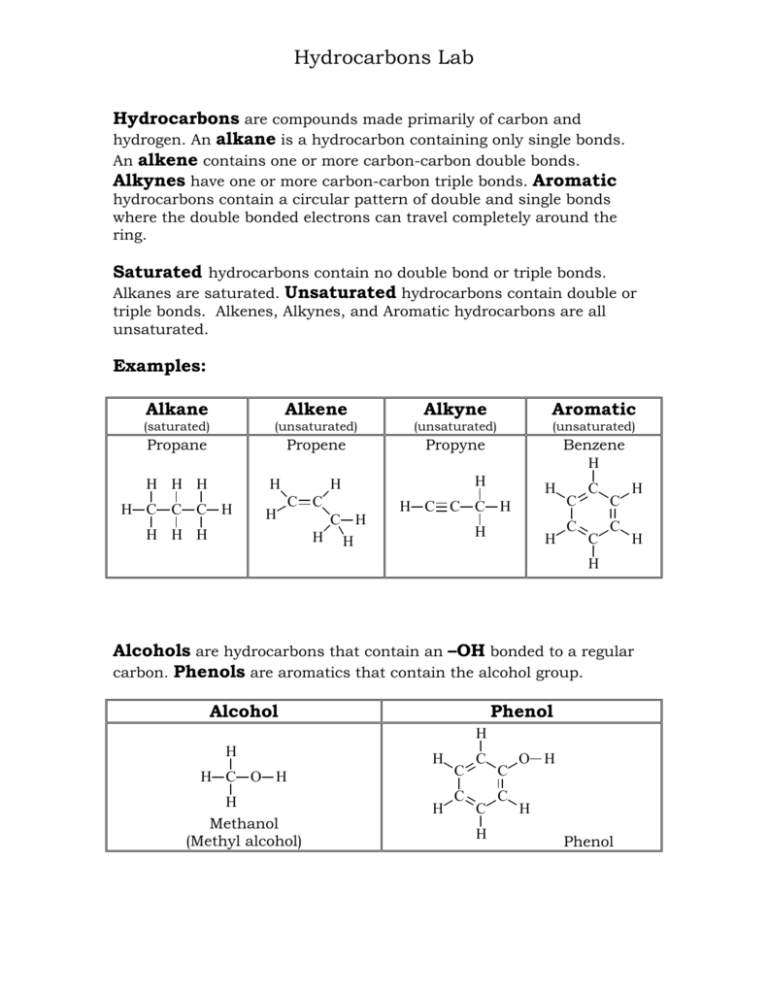
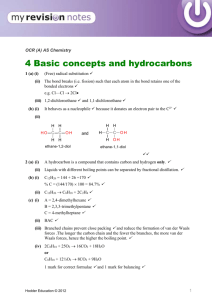
Hydrocarbons Lab Hydrocarbons are compounds made primarily of carbon and hydrogen. An alkane is a hydrocarbon containing only single bonds. An alkene contains one or more carbon-carbon double bonds. Alkynes have one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain a circular pattern of double and single bonds where the double bonded electrons can travel completely around the ring. Saturated hydrocarbons contain no double bond or triple bonds. Alkanes are saturated. Unsaturated hydrocarbons contain double or triple bonds. Alkenes, Alkynes, and Aromatic hydrocarbons are all unsaturated. Examples: Alkane Alkene Alkyne Aromatic (saturated) (unsaturated) (unsaturated) (unsaturated) Propane Propene Propyne Benzene H H H H H H C C C H H H H C C H H H C H H H H H C C C H H H C C C C C C H H H Alcohols are hydrocarbons that contain an –OH bonded to a regular carbon. Phenols are aromatics that contain the alcohol group. Alcohol Phenol H H H H C O H H Methanol (Methyl alcohol) H C C C C H C C O H H Phenol 2 Physical Properties: Volatility: Because of the small difference in electronegativities between carbon (C=2.5) and hydrogen (H=2.1), hydrocarbons are characteristically nonpolar. Since hydrocarbons do not have partially negative or partially positive atoms there is minimal attraction between hydrocarbon molecules. Thus, hydrocarbons require less energy to evaporate or vaporize than do polar compounds. Small hydrocarbons require less energy to vaporize than large ones. They are highly volatile. Odor: The volatile nature of hydrocarbons causes them to vaporize and move through the air. These air-borne molecules reach our noses and we smell them. Many hydrocarbons have characteristic odors. Solubility: The nonpolar character of hydrocarbons allows them to have minimal attraction to other hydrocarbons but causes them to repel polar compounds such as water. Density: Nonpolar hydrocarbons have minimal attraction to each other so hold together loosely. They have low density. Polar compounds, on the other hand, attract each other and bind close. Water has a lot of hydrogen bonding and is particularly dense.) Chemical Properties: Combustion: Hydrocarbons easily combust in the presence of oxygen. They are commonly used for fuel. Wood, fuel oil, gasoline, diesel, and candle wax are all common flammable hydrocarbons fuels. CH4 methane + 2O2 CO2 oxygen carbon dioxide + 2H2O water Not all hydrocarbons will react with the same amount of oxygen so when burning in a normal atmosphere they may appear very different. Aromatic hydrocarbons, for example, burn very dirty in that they undergo incomplete combustion and produce a lot of soot. Small hydrocarbons and alcohols are more likely to completely combust and burn cleanly, with very little or no soot. 3 Bromination: The double bond of an alkene reacts with halogens to form alkyl halide compounds. The pi bond (second bond) of the double bond is weak and breaks more easily than the sigma bond (first bond). H H C C H Br2 + CH3 propene (colorless) bromine (brown) Br Br H C C H CH3 H 1,2-dibromopropane (colorless) Bromine (Br2) is a brown liquid so if a reaction occurs in which the Br2 splits apart and the Br’s bond with carbons we will see the brown color disappear. Aromatic compounds do not react with halogens in the same way. The double bonds of an aromatic compound are stronger and so do not easily break. Oxidation: Some hydrocarbons are easily oxidized with potassium permanganate, KMnO4. H H + C C H KMnO4 CH3 potassium permanganate (purple) propene (colorless) HO OH H C C H CH3 H + MnO2 manganese (IV) oxide (brown) 1,2-propandiol (colorless) Potassium Permanganate is purple so if a reaction occurs in which the KMnO4 oxidizes the carbons we will see the purple KMnO4 change to brown MnO2. 4 Procedures: I. PHYSICAL PROPERTIES: A. Volatility: 1. Obtain three small beakers of identical size. Label them #1, #2, #3. 2. Into beaker #1 put 5 mL of water. Into beaker #2 put 5 mL of ethanol. Into beaker #3 put 5 mL of hexane. 3. Carefully weigh each beaker on the electronic balance and record the masses on your report sheet. 4. Weigh each beaker again every 15 minutes and record the masses. 5. On the report sheet make a graph of the total mass lost by each substance every 15 minutes. Draw a straight line through the graph points to show the linear relationship for the evaporation of each liquid. Compare to determine relative volatility. B. Solubility in Water: 1. Obtain 4 stoppered test tubes, each containing 2 mLs of water. 2. Into tube #1 put 1 mL ethanol. (C2H5OH) Into tube #2 put 1 mL of hexane. (C6H14) Into tube #3 put 1 mL cyclohexene. (C6H10) Into tube #4 put 1 mL toluene. (C6H5CH3) Stopper the tubes and shake each to mix. 3. Check each tube for layers. Record your observations about the solubility of each hydrocarbon in water. Use ‘S’ for soluble, ‘PS’ for partially soluble, and ‘I’ for insoluble. Save these tubes for use in Part IC. 5 C. Density: 4. For each insoluble hydrocarbon in Part IB observe the positioning of the hydrocarbon layer relative to water. The more dense substance will be on the bottom. 5. Record the density of each hydrocarbon relative to water on the report sheet. Report M for more dense than water, L for less dense than water, and X if there is not enough evidence to determine. 6. Dispose of hydrocarbons in the designated waste containers. D. Solubility in Other Hydrocarbons: 1. Obtain 3 dry stoppered test tubes, each containing 1 mL of hexane (C6H14). 2. Into tube #1 put 1 mL ethanol. (C2H5OH) Into tube #2 put 1 mL cyclohexene. (C6H10) Into tube #3 put 1 mL toluene. (C6H5CH3) Stopper the tubes and shake each to mix. 3. Check each tube for layers. Record your observations about the solubility of each hydrocarbon in hexane (a typical hydrocarbon solvent). Dispose of hydrocarbons in the designated waste containers. II. CHEMICAL PROPERTIES: A. Combustion: 1. Line up 4 clean and dry watch glasses about 6 inches apart in a fume hood. 2. Onto Onto Onto Onto watch watch watch watch glass glass glass glass #1 #2 #3 #4 put put put put 10 10 10 10 drops drops drops drops ethanol. (C2H5OH) of hexane. (C6H14) cyclohexene. (C6H10) toluene. (C6H5CH3) 3. Quickly go down the row and ignite each liquid with a burning wood splint and compare the colors and types of flames produced by each hydrocarbon as they burn together. Record your observations. 6 B. Bromination: 1. Obtain 4 dry stoppered test tubes. 2. Into Into Into Into tube tube tube tube #1 #2 #3 #4 put put put put 1 1 1 1 mL mL mL mL ethanol. (C2H5OH) hexane. (C6H14) cyclohexene. (C6H10) toluene. (C6H5CH3) 3. Move your tubes to a fume hood and into each tube drop 3 drops of Bromine (Br2) solution. Swirl to mix if needed and record any results. 4. Dispose in the designated waste containers. Tubes containing bromine must go into “bromine waste”. C. Oxidation: 1. Obtain 4 clean stoppered test tubes. 2. Into Into Into Into tube tube tube tube #1 #2 #3 #4 put put put put 1 1 1 1 mL mL mL mL ethanol. (C2H5OH) hexane. (C6H14) cyclohexene. (C6H10) toluene. (C6H5CH3) 3. Into each tube drop 3 drops of Potassium Permanganate (KMnO4) solution. Stopper and shake to mix well. Record any results. Dispose in the designated waste containers. 7 Name Date Report for Experiment: Hydrocarbons I. PHYSICAL PROPERTIES: A. Volatility: a. Mass at Start b. Mass lost in zero min c. Mass at 15 minutes d. Total g’s lost from beginning e. Mass at 30 minutes f. Total g’s lost from beginning g. Mass at 45 minutes h. Total g’s lost from beginning i. Mass at 60 minutes j. Total g’s lost from beginning #1 + Water #2 + Ethanol #3 + Hexane Mass in grams Mass in grams Mass in grams Og Og Og (a-c) (a-c) (a-c) (a-e) (a-e) (a-e) (a-g) (a-g) (a-g) (a-i) (a-i) (a-i) Mass lost by Water (#1), Ethanol (#2), and Hexane (3#) Total Mass Lost From the Beginning (in grams) 0 15 (a-c) 30 45 (a-e) Time (a-g) 60 (a-i) (in minutes) Volatility Results Summary: Rank the substances in order of decreasing volatility. Most volatile > > Least volatile 8 Ethanol Hexane Cyclohexene Toluene An Alcohol An Alkane An Alkene An Aromatic B. Solubility in Water C. Density Compared to Water D. Solubility in Hydrocarbons Physical Properties Results Summary: 1. As A. B. C. a general rule, most hydrocarbons are _____ than water. more volatile less volatile there is no general rule 2. As a general rule, hydrocarbons are _____ in water and _____in other hydrocarbons. A. soluble, soluble B. insoluble, soluble C. insoluble, insoluble D. soluble, insoluble 3. As A. B. C. a general rule, most hydrocarbons are _____ than water. more dense less dense there is no general rule II. CHEMICAL REACTIVITY: A. Combustion B. Bromination (reaction w/ Br2) C. Oxidation (reaction w/ KMnO4) Ethanol Hexane Cyclohexene Toluene An Alcohol An Alkane An Alkene An Aromatic