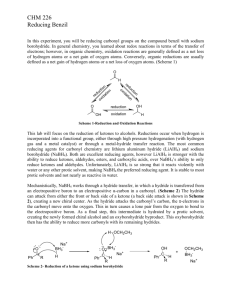
Dilantin® from Benzaldehyde
advertisement
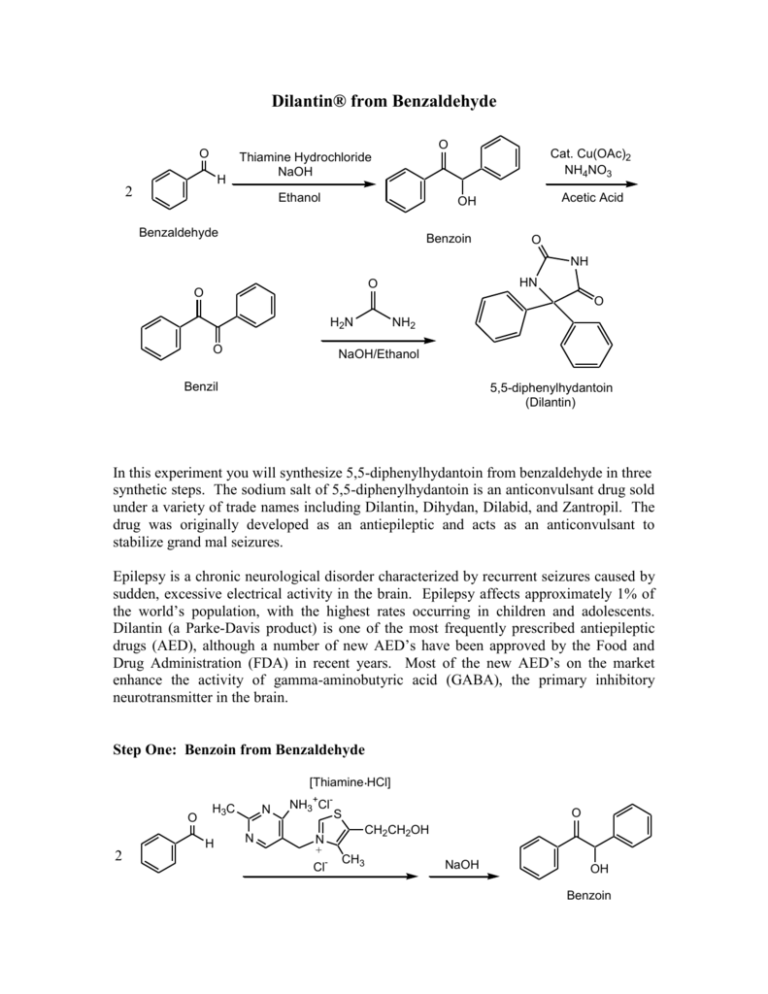
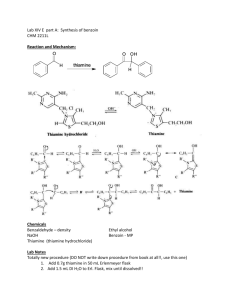
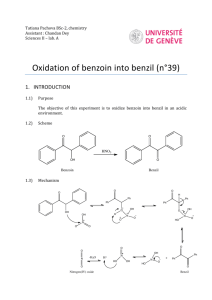
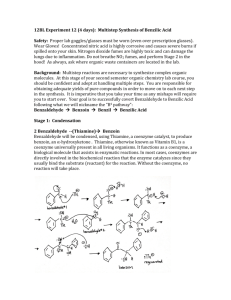
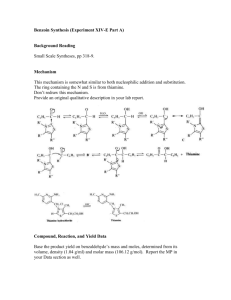
Dilantin® from Benzaldehyde O O H 2 Cat. Cu(OAc)2 NH4NO3 Thiamine Hydrochloride NaOH Ethanol Acetic Acid OH Benzaldehyde Benzoin O NH HN O O O H2N O NH2 NaOH/Ethanol Benzil 5,5-diphenylhydantoin (Dilantin) In this experiment you will synthesize 5,5-diphenylhydantoin from benzaldehyde in three synthetic steps. The sodium salt of 5,5-diphenylhydantoin is an anticonvulsant drug sold under a variety of trade names including Dilantin, Dihydan, Dilabid, and Zantropil. The drug was originally developed as an antiepileptic and acts as an anticonvulsant to stabilize grand mal seizures. Epilepsy is a chronic neurological disorder characterized by recurrent seizures caused by sudden, excessive electrical activity in the brain. Epilepsy affects approximately 1% of the world’s population, with the highest rates occurring in children and adolescents. Dilantin (a Parke-Davis product) is one of the most frequently prescribed antiepileptic drugs (AED), although a number of new AED’s have been approved by the Food and Drug Administration (FDA) in recent years. Most of the new AED’s on the market enhance the activity of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the brain. Step One: Benzoin from Benzaldehyde [Thiamine HCl] O 2 H3C H N N NH3+ClN + Cl- O S CH2CH2OH CH3 NaOH OH Benzoin Benzaldehyde does not possess alpha hydrogens and therefore does not undergo a selfaldol condensation. In the presence of a catalyst such as thiamine, however benzaldehyde undergoes a unique self-condensation reaction, called the benzoin condensation. The product is the -hydroxy ketone called benzoin. The reaction proceeds via sequential nucleophilic additions to the carbonyl groups of the two benzaldehyde molecules and it is catalyzed by the ylide of thiamine hydrochloride in aqueous base. The formation of the ylide ion is illustrated below. S H S CH2CH2OH CH2CH2OH NaOH N NaCl / H2O N +- CH3 R Cl + R N H3C R= N NH2 CH2- CH3 Ylide The ylide acts as a nucleophile and attacks the electropositive carbon of the C=O of benzaldehyde. This generates intermediate I (For simplicity the ylide shown above is represented by X- in the following scheme). O- O H X OH H-O-H C-H C-H X X - OH- (I) O O- X OH H OH C + H2O X O O H Benzoin The benzylic hydrogen of intermediate I is acidic enough to be removed by the base present in the reaction. Next, this conjugate base of intermediate I acts as a nucleophile and attacks the electropositive carbon of another C=O of benzaldehyde. Finally, a proton exchange and elimination of the ylide anion produce benzoin. EXPERIMENTAL PROCEDURE It is important that the benzaldehyde be free from any benzoic acid. If the bottle of benzaldehyde you are to use for the reaction appears to have any solids (benzoic acid) in it, don’t use it. Ask the instructor for another sample of benzaldehyde. Dissolve 1.2 g of thiamine hydrochloride in about 3.3 mL of distilled water in a 50 mL Erlenmeyer flask. Add 10 mL of 95% ethanol and cool the solution by swirling the flask in an ice-water bath. Meanwhile, place about 3.3 mL of 2 M sodium hydroxide solution in a small Erlenmeyer flask. Cool this solution in the ice bath as well. Then, over a period of about 10 minutes, add the cold sodium hydroxide solution to the thiamine solution. Weigh 6.7 mL of benzaldehyde and add it to the reaction mixture. Cork the flask and wrap parafilm around the cork. Allow the mixture to sit at room temperature for one week. (Be sure to label your flask). After cooling in an ice bath, collect the product by vacuum filtration using a Buchner funnel.* Wash the product with two 17 mL portions of cold water. Weigh the crude product and then recrystallize it from 95% ethanol. The solubility of benzoin in boiling 95% ethanol is about 12 to 14 g per 100 mL. Weigh the product, calculate the percentage yield, and determine its melting point. * If the benzoin forms an oil on cooling, scratch the solution and cool further. If this does not induce crystallization heat the solution to redissolve the benzoin oil and then cool more slowly. Step Two. Benzil from Benzoin O O Cat. Cu(OAc)2 NH4NO3 OH Bezoin Acetic Acid O Benzil In the second step of this multistep synthesis the -diketone, benzil, will be prepared through the oxidation of the -hydroxyketone, benzoin. This oxidation may be easily done using mild oxidizing reagents such as Fehling’s solution (alkaline cupric tartrate), palladium (II) acetate in dimethylsulfoxide under an oxygen atmosphere, copper sulfate in pyridine, and nitric acid. We will perform this oxidation with a solution of ammonium nitrate in the presence of a catalytic amount of copper (II) acetate. The conversion of benzoin to benzil is a catalytic oxidation reaction. The benzoin is oxidized to benzil by the copper (II) ions, which are reduced to copper (I) ions in the process. The catalyst for the reaction is then regenerated when the ammonium nitrate oxidizes the copper (I) ions back to copper (II) ions. During the oxidation of the copper (I) ions, the ammonium nitrate is reduced to nitrogen gas and water. EXPERIMENTAL PROCEDURE First, weigh out 1.0 g of benzoin and place it into a dry 25mL round bottom flask. Carefully add 2.7 mL of glacial acetic acid to the flask. Next, add 0.47 g of ammonium nitrate and 1.0 mL of a 2% (wt./wt.) aqueous solution of Cu(OAc)2H2O. Place a boiling stone in the round bottom flask and attach a water-jacketed condenser. Keep a slow, steady flow of water circulating through the condenser at all times while heating. Warm the reaction to gentle reflux using the heating mantle. Maintain a steady reflux for 90 minutes. After refluxing the reaction for 90 minutes, remove the round bottom flask from the heating mantle and allow it to cool for 5 minutes. Pour the reaction mixture into a 30 mL beaker containing 5-7 mL of ice water. Stir the resulting mixture with your glass stirring rod for 5 minutes. Try to break up the hard, yellow solids that are precipitated. Isolate the yellow product using vacuum filtration through your Buchner funnel. Wash the benzil product with small aliquots of ice water. Dry the product with aspiration through the Buchner funnel for 5-10 minutes. Place the benzil product in a clean beaker and allow it to dry until the next laboratory period. Obtain a mass, percent yield, and a melting point for your benzil product at the beginning of the next lab period. Questions 1. What major differences would be observed in the IR spectra of benzoin and benzil? 2. What structural features make benzil yellow and benzoin colorless (white)? 3. Would you have obtained the same results for the oxidation of benzoin if the label on the copper acetate bottle had read “cuprous acetate?” Step Three. Dilantin from Benzil O NH HN O O O H2N O Benzil NH2 NaOH/Ethanol 5,5-diphenylhydantoin (Dilantin) In the final step of this multistep synthesis, you will prepare Dilantin® by treating benzil with urea in the presence of the base (NaOH). The mechanism of this reaction begins with the nucleophilic attack of a urea nitrogen atom on one of the carbonyls of benzil. O O O O- O- HO O - N-C-NH2 OH N-C-NH2 + Ph Ph Ph O H2N Ph Ph H NH2 Ph Ph Benzylic acid Rearrangement Ph O Ph H2O OH HO NH H2N O O O HO N-C-NH2 NH = OH2N O - Ph Ph Ph O Dehydration Ph O Ph NH HN O 5,5-diphenylhydantoin (Dilantin) The loss of a proton from the quaternary nitrogen atom produces an intermediate containing a ketone and an oxyanion. Then, this intermediate undergoes the classic benzilic acid rearrangement discovered by von Liebig. In this reaction, the benzilic acid rearrangement precedes through a 1,2-phenyl migration to produce an amide and a tertiary alkoxide in the resulting intermediate. Finally, dehydration produces the 5,5diphenylhydantoin product. EXPERIMENTAL PROCEDURE Because Dilantin® has significant biological activity, it is very important that protective gloves and safety goggles be worn when handling the product of this reaction! Weigh out 500 mg of benzil into a dry, tared 25 mL round bottom flask. Add 7.2 mL of 95% ethanol and swirl the round bottom flask until the benzil dissolves. Then, add 0.25 g of urea and 1.3 mL of 30% (wt./wt.) aqueous solution of NaOH. Place a boiling stone into the flask, and attach a water-jacketed condenser. Keep a slow, steady flow of water circulating through the condenser at all times while heating. Warm the reaction to a gentle reflux using a heating mantle. Maintain reflux for at least 1 hour. After refluxing for an hour, remove the reaction flask from the heating mantle, and allow the reaction mixture to cool to room temperature. Now, add 12 mL of distilled water to the round bottom flask. If solids are present, remove these solids by vacuum filtration through a Buchner funnel. SAVE THE FILTRATE!!! Dispose of the solids in the recovered organic solids container. Pour the filtrate into a 30 mL or 50 mL beaker, and slowly add concentrated HCl dropwise until the pH of the filtrate is 2-3. Cool the resulting acidic solution in an ice water bath until all of the product has precipitated. Collect the product by vacuum filtration through your Buchner funnel. Wash the product with small aliquots of ice water. Dry the Dilantin® product with aspiration through the Buchner funnel for 5 – 10 minutes, then obtain a mass, percent yield, and a melting point for your 5,5-diphenylhydantoin product. QUESTIONS 1. Write an equation that illustrates why 5,5-diphenylhydantoin precipitates when the reaction mixture is treated with concentrated HCl. 2. Barbiturates are another example of biologically important heterocyclic compounds. The diethyl compound shown below (Barbital) is a sedative and hypnotic. It is synthesized by the reaction of urea and the diethyl ester of ,diethylmalonic acid in the presence of an alkoxide base. Propose a stepwise mechanism for this reaction. O H N O NH O Barbital Cleaning up. The filtrates resulting from the isolation of the crude product and recrystallization of the product should be placed in the aqueous recovery bottle. Your Dilantin® product should be placed in the Dilantin® recovery bottle.