John Congleton - Orange Coast College
advertisement

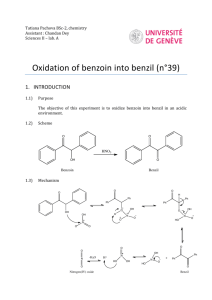
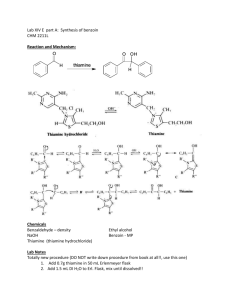
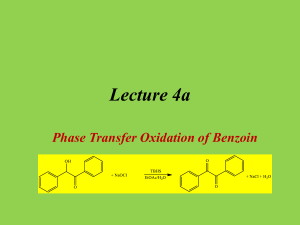
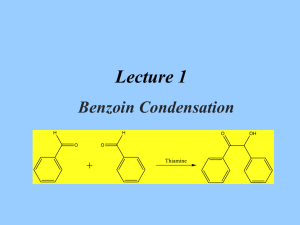
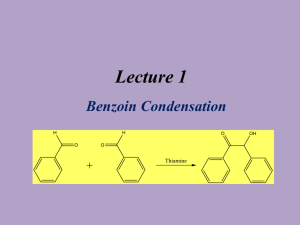
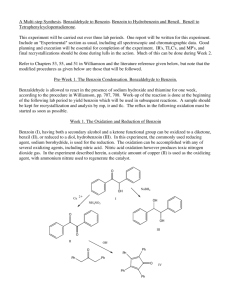
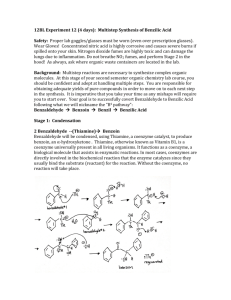
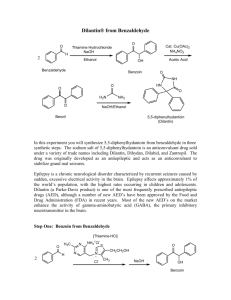
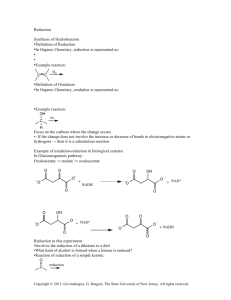
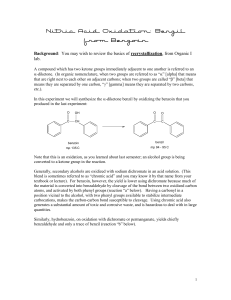
Multistep Synthesis of Benzil: Introduction This experiment will have two separate reactions. First we will synthesize, purify, isolate and characterize benzoin and then we will use our prepared benzoin to synthesize, purify, isolate and characterize our final product benzil. Reading Pavia, et al. 3rd edition, Expt. 32, pp. 302–310. Pavia, et al. 3rd edition , Expt. 33, pp. 310–312 Prelab Assignment Your prelab should include the following: Date, Introduction, Haz-Mat for Part A and B, Reactant Tables for Part A and Part B, Chemical Equations for Part A and Part B, and Procedures. Procedural Notes on Part A Isolation of Crude Benzoin: If the reaction mixture has not formed a solid after being left for two days, try seeding the reaction. Coolect crystals via Hirsch funnel and rinse the crude product with ice-cold water, then let it dry on the Hirsch funnel. Don’t forget to weigh the crude product before recrystallizing (we can get a rough idea of how much hot EtOH to use in the recrystallization…). Yield Calculation and MP Determination: We are not going to calculate the crude MP or percent yield of the crude. We will recrystallize and then calculate percent recovery and percent yield. Crystallization of Benzoin: After filtering the recrystallized product, rinse the crystals with small portions (about o 0.5–1.0 mL) of ice-cold ethanol. The purified product can be dried in a 100 C oven for about 15 min before determining the final weight, the melting point, and the IR spectrum. Alternately, you can try to air dry the product via vacuum in your Hirsch funnel for ~10 minutes. Keep your product for the next experiment. NOTE: We will use 0.300 g of our purified product in the next experiment. Procedural Notes for Part B We will follow the procedure in the lab book. Please note the following: 1. We will use the purified product from the benzoin synthesis as the reactant in this experiment. Students who have less than 0.300 g of benzoin will be given additional benzoin by the instructor. 2. The reaction gives off a noxious brown gas (NO2 and N2O4- Yuck! SMOG!) during the heating. We will use the bench top fume hoods to contain the fumes. Make sure to lower the bell of the hood so that the top of the condenser is above the bottom rim of the bell. 3. Reminder: Remember to always stand the conical vials in a small beaker to avoid them falling over and spilling. Postlab Questions Part A Answer these questions in your lab notebook after your conclusion is a section titled “Post-lab Questions” 1. What is the impurity that must be removed from the benzaldehyde reagent before it can be used in this experiment? 2. 3. 4. Refer to the essay that precedes the experiment. It gives the structure of thiamine pyrophosphate. Draw a structure of thiamine hydrochloride. What is the purpose of adding NaOH ot the reaction? Using the information given in the essay that proceeds this experiment, formulate a complete mechanism for the thiamine-catalyzed conversion of benzaldehyde to benzoin. © John Congleton 5. What modifications of conditions would be appropriate if an enzyme was used for this reaction? Postlab Question Part B Answer these questions in your lab notebook after your conclusion is a section titled “Post-lab Questions” o 1. The instructions in the lab text emphasize that the reaction mixture should not be heated above 70 C. According to the lab text, what could happen if the reaction temperature is too high? 2. Compare the IR spectrum of benzoin (on page 309 of the lab text) with the IR spectrum of benzil (page 312). Explain how the changes in the important peaks are related to the differences between the two structures. © John Congleton