Calculation of X-ray wavelengths
advertisement
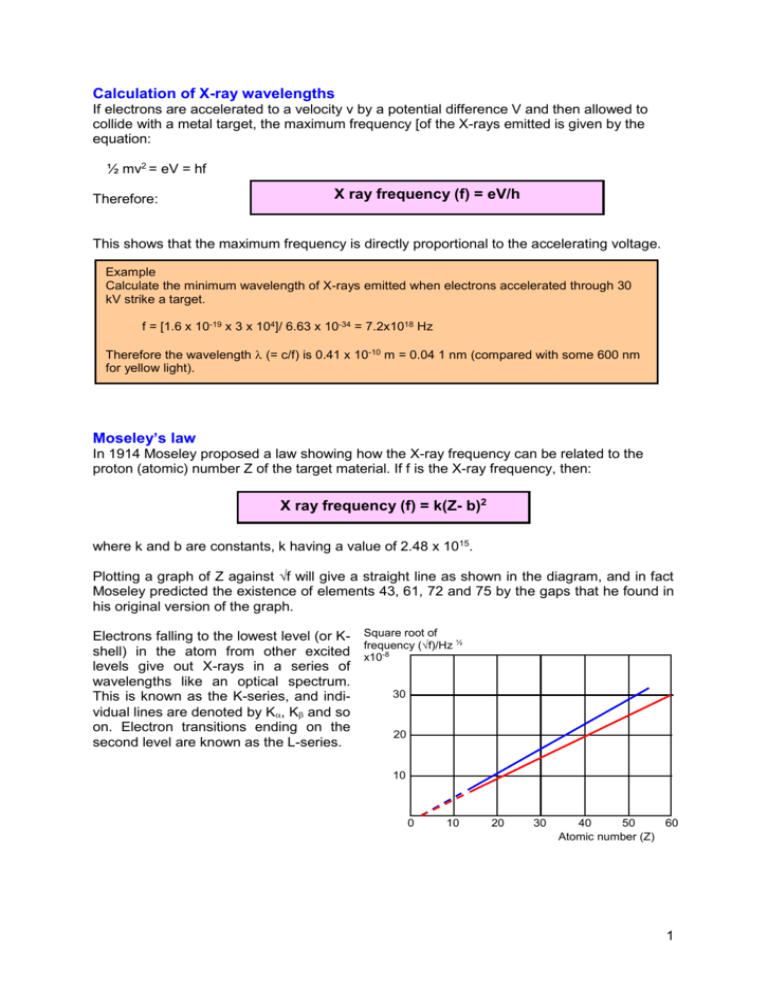
Calculation of X-ray wavelengths If electrons are accelerated to a velocity v by a potential difference V and then allowed to collide with a metal target, the maximum frequency [of the X-rays emitted is given by the equation: ½ mv2 = eV = hf Therefore: X ray frequency (f) = eV/h This shows that the maximum frequency is directly proportional to the accelerating voltage. Example Calculate the minimum wavelength of X-rays emitted when electrons accelerated through 30 kV strike a target. f = [1.6 x 10-19 x 3 x 104]/ 6.63 x 10-34 = 7.2x1018 Hz Therefore the wavelength (= c/f) is 0.41 x 10-10 m = 0.04 1 nm (compared with some 600 nm for yellow light). Moseley’s law In 1914 Moseley proposed a law showing how the X-ray frequency can be related to the proton (atomic) number Z of the target material. If f is the X-ray frequency, then: X ray frequency (f) = k(Z- b)2 where k and b are constants, k having a value of 2.48 x 1015. Plotting a graph of Z against √f will give a straight line as shown in the diagram, and in fact Moseley predicted the existence of elements 43, 61, 72 and 75 by the gaps that he found in his original version of the graph. Electrons falling to the lowest level (or K- Square root of ½ frequency (√f)/Hz shell) in the atom from other excited x10-8 levels give out X-rays in a series of wavelengths like an optical spectrum. 30 This is known as the K-series, and individual lines are denoted by K, K and so on. Electron transitions ending on the 20 second level are known as the L-series. 10 0 10 20 30 40 50 60 Atomic number (Z) 1 The following table shows the wavelengths of the K, lines for some elements: Element Aluminium Calcium Manganese Iron Cobalt Nickel Proton number 13 20 25 26 27 28 Wavelength (nm) 0.823 0.335 0.210 0.194 0.179 0.166 Element Copper Bromine Silver Tungsten Uranium Proton number 29 35 47 74 92 Wavelength (nm) 0.139 0.104 0.056 0.021 0.017 2