Name _________________ Test 2 October 18, 2013
advertisement
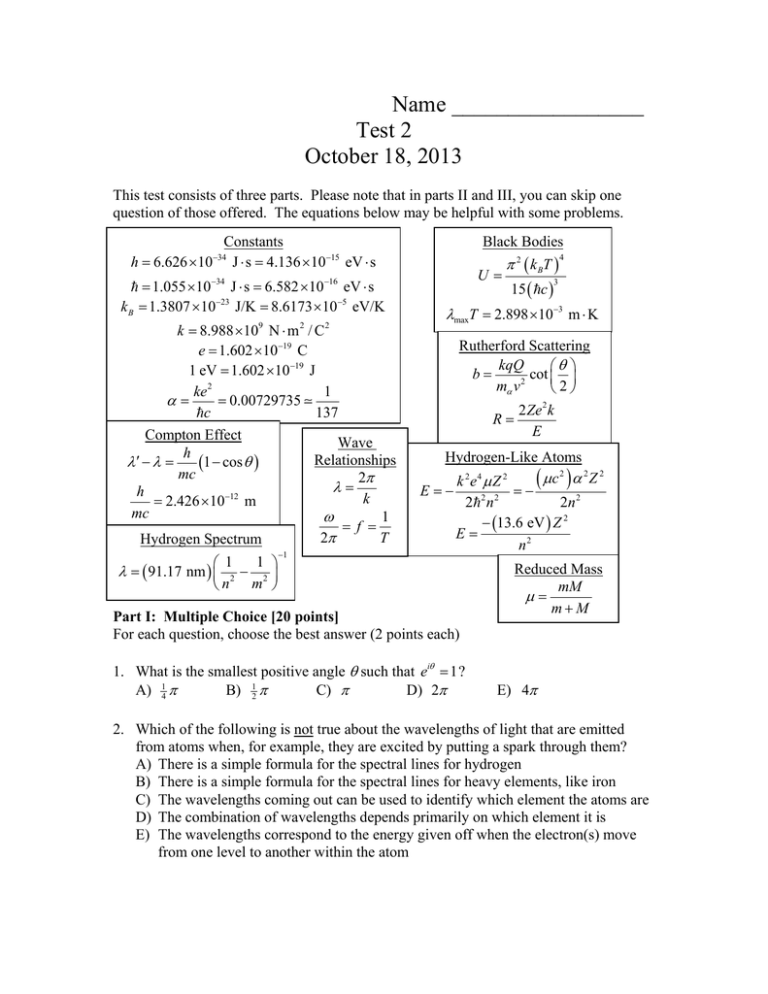
Name _________________ Test 2 October 18, 2013 This test consists of three parts. Please note that in parts II and III, you can skip one question of those offered. The equations below may be helpful with some problems. Constants h 6.626 10 J s 4.136 1015 eV s Black Bodies 34 1.055 1034 J s 6.582 1016 eV s k B 1.3807 1023 J/K 8.6173 105 eV/K k 8.988 10 N m / C e 1.602 1019 C 1 eV 1.602 1019 J ke 2 1 0.00729735 137 c Compton Effect Wave h Relationships 1 cos mc 2 h 12 k 2.426 10 m mc 1 f 2 T Hydrogen Spectrum 9 1 1 2 2 n m 91.17 nm 2 1 2 U 2 k BT 15 c 4 3 maxT 2.898 103 m K Rutherford Scattering kqQ cot b 2 m v 2 R 2Ze 2 k E Hydrogen-Like Atoms c2 2 Z 2 k 2e4 Z 2 E 2 2 n 2 2n 2 13.6 eV Z 2 E n2 Part I: Multiple Choice [20 points] For each question, choose the best answer (2 points each) 1. What is the smallest positive angle such that ei 1 ? A) 14 B) 12 C) D) 2 Reduced Mass mM mM E) 4 2. Which of the following is not true about the wavelengths of light that are emitted from atoms when, for example, they are excited by putting a spark through them? A) There is a simple formula for the spectral lines for hydrogen B) There is a simple formula for the spectral lines for heavy elements, like iron C) The wavelengths coming out can be used to identify which element the atoms are D) The combination of wavelengths depends primarily on which element it is E) The wavelengths correspond to the energy given off when the electron(s) move from one level to another within the atom 3. If we have a perfect black body distribution, if we double the temperature, the energy density is multiplied by a factor of ___ and the peak wavelength by a factor of ____ A) 2, 2 B) 2, ½ C) 16, 2 D) 16, ½ E) ½, ½ 4. Which types of objects, under appropriate circumstances, can sometimes act like waves and sometimes like particles? A) Photons (only) B) Electrons (only) C) Atoms (only) D) Photons and electrons, but not atoms E) Photons, electrons, and atoms 5. The reason you can get very strong X-ray scattering at certain angles with certain wavelengths from a crystal is because A) There is constructive interference adding together waves scattering from many layers of the atoms B) These angles are the angles that the electrons are aligned along inside the atoms C) These X-rays have the exact right energy to be absorbed and then re-emitted from the crystals D) The wavelength of the X-rays match exactly the wavelength of the electrons in the crystal E) The angles are those so that the X-rays can perfectly slip between atoms without ever bumping into a nucleus 6. If an atom were the size of the circle at right, how large would the nucleus be? A) B) C) D) E) Smaller than those 7. The momentum of a particle with wavelength is given by B) h C) h D) E) A) h 8. In the Franck-Hertz experiment, increasing the voltage accelerating electrons through a thin gas causes the current to increase, until a certain threshold is reached, when there is a sudden decrease in the current. What is causing this decrease? A) When the electrons reach this energy, they start producing electron-positron pairs, which slows them down B) The electrons have grown so energetic they actually quantum tunnel through the collector plate C) The electrons have enough energy to bump up atoms they collide with to a higher energy, so the electrons can now lose energy D) The high voltage makes their momentum high, and therefore very definite, making the actual position of the electrons highly uncertain E) The voltages are so strong, the thin gas starts moving, which is much slower than the electrons 9. Which of the following is a consequence of one of the classical uncertainty principles? A) A sufficiently short musical note does not have a definite pitch B) An object that is constrained in a box of size L has a position uncertainty of about 1 4 L C) It is never possible to know very precisely the momentum of a particle D) The process of measuring a particle’s wavelength changes that wavelength E) I am entirely uncertain of the answer to this question, so mark it wrong 10. Which of the following best explains why the harmonic oscillator has a minimum energy that is greater than zero? A) Because a particle is a wave, its momentum cannot be exactly zero B) Because a particle is a wave, its position cannot be exactly zero C) Because of the uncertainty principle, the position and momentum cannot simultaneously forced to be zero, their minimum value D) Because of the uncertainty principle, the energy cannot be precisely known in finite time E) The process of measuring the harmonic oscillator forces its energy to become positive Part II: Short answer [20 points] Choose two of the following questions and give a short answer (2-3 sentences) (10 points each). 11. Light with frequency f shines on a metal. Explain under what conditions it will manage to dislodge an electron from the metal, and if it does, how much voltage V the resulting electron will be able to go against before being stopped. At least one formula is highly advised. 12. Explain qualitatively (or with a picture) the difference between group velocity and phase velocity. For a quantum wave packet (like an electron), which is the speed of the electron? 13. What did Rutherford learn by scattering -particles off of atoms? Also, what in particular was he able to measure by using the highest energy -particles available and using atoms with small total positive charge Z? Part III: Calculation: [60 points] Choose three of the following four questions and perform the indicated calculations (20 points each). 14. An experimenter is scattering X-rays of unknown wavelength off of electrons, and measuring the wavelength when they scatter to an arbitrary angle , as sketched above. At an angle of 90 , the observed wavelength is 1.083 1011 m . (a) What is the incoming wavelength ? (b) At what angle would the observed scattered wavelength be 1.204 1011 m ? (c) What is the frequency for the incoming X-rays? What is the energy in eV? 15. Pionic hydrogen consists of a proton (mp = 938.3 MeV/c2) electromagnetically bound to a meson (m = 139.6 MeV/c2). Except for the replacement of the electron with a pion, it is otherwise just like ordinary hydrogen. (a) What is the reduced mass for this system, preferably in MeV/c2? (b) What is the energy of the n’th state of a pion bound in pionic hydrogen? (c) If the pion fell from the n = 20 to n = 17 state, what would be the energy, in eV, of the resulting photon that would be emitted? (d) Find the wavelength in nm of the photon you found in part (c). 16. A proton is believed to have three objects called quarks inside of them. A proton may be thought of as a bag approximately 1.76×10–15 m in diameter, inside which must be found the quarks. (a) According to Carlson’s rule, what is the approximate uncertainty in the position of the quark? (b) What is the corresponding minimum uncertainty in the momentum of the quark? (c) Using non-relativistic formulas, find the corresponding uncertainty in the velocity of the quark in m/s. Assume a quark has a mass mq 13 m p 5.58 1028 kg . (d) Find v c . If you followed directions in part (c), you used non-relativistic formulas. Do you think this was a good idea? Why or why not? 17. The wave function for a particle in the n = 3 state of the infinite square well has wave function N sin 3 x a 0 x a, otherwise . x 0 where N and a are positive constants. The wave is sketched at right. Possibly helpful integrals are listed below. (a) Based on the graph estimate the most likely values of x/a to find the particle. You are not expected to do any calculations. (b) What is the normalization constant N? (c) If the particle’s position is measured, what is the probability that it will be found to have a value in the range 0 x 14 a ? sin x dx cos x , Indefinite Integrals: cos x dx sin x , 1 1 sin x dx cos x dx 2 1 2 x 41 sin 2 x , 2 1 2 x 41 sin 2 x .