hw14 - Iowa State University
advertisement
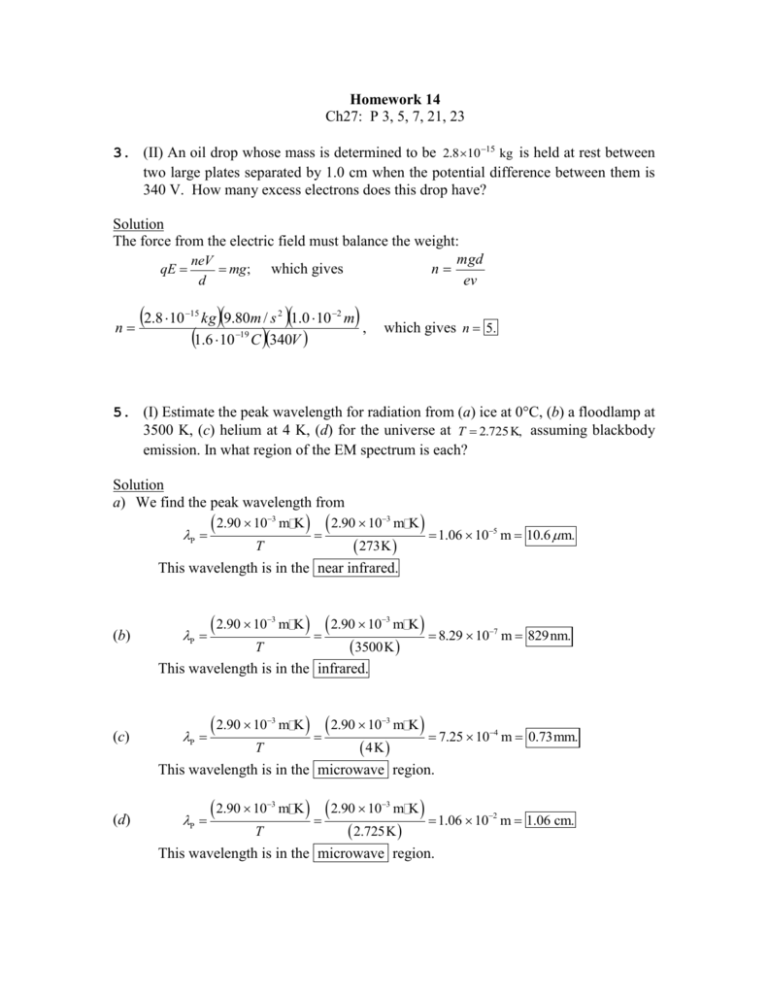
Homework 14 Ch27: P 3, 5, 7, 21, 23 3. (II) An oil drop whose mass is determined to be 2.8 10 15 kg is held at rest between two large plates separated by 1.0 cm when the potential difference between them is 340 V. How many excess electrons does this drop have? Solution The force from the electric field must balance the weight: mgd neV n qE mg ; which gives d ev n 2.8 10 15 kg 9.80m / s 2 1.0 10 2 m , 1.6 10 19 C 340V which gives n 5. 5. (I) Estimate the peak wavelength for radiation from (a) ice at 0°C, (b) a floodlamp at 3500 K, (c) helium at 4 K, (d) for the universe at T 2.725 K, assuming blackbody emission. In what region of the EM spectrum is each? Solution a) We find the peak wavelength from P 2.90 10 3 m K T 2.90 10 3 m K 273K 1.06 105 m 10.6 m. This wavelength is in the near infrared. (b) P 2.90 10 3 m K T 2.90 10 3 m K 3500K 8.29 107 m 829nm. This wavelength is in the infrared. (c) P 2.90 10 3 m K T 2.90 10 3 4K m K 7.25 104 m 0.73mm. This wavelength is in the microwave region. (d) P 2.90 10 T 3 m K 2.90 10 3 m K 2.725K 1.06 102 m 1.06 cm. This wavelength is in the microwave region. 7. (I) An HCl molecule vibrates with a natural frequency of 8.1 1013 Hz. What is the difference in energy (in joules and electron volts) between possible values of the oscillation energy? Solution Because the energy is quantized, E = nhf, the difference in energy between adjacent levels is E hf 6.63 1034 J s 8.1 1013Hz 5.4 1020 J 0.34eV. 21. (II) What is the maximum kinetic energy of electrons ejected from barium (W0 2.48 eV) when illuminated by white light, 400 to 750 nm? Solution The photon of visible light with the maximum energy has the minimum wavelength: hf max hc min 6.63 10 160 10 34 19 J s 3.00 108 m / s J/eV 400 109 m 3.11 eV. The maximum kinetic energy of the photoelectrons is KE max hf W0 3.11 eV 2.48 eV 0.63 eV. 23. (II) When UV light of wavelength 285 nm falls on a metal surface, the maximum kinetic energy of emitted electrons is 1.40 eV. What is the work function of the metal? Solution The energy of the photon is hf hc 6.63 10 1.60 10 34 19 J s 3.00 108 m / s J/eV 285 109 m 4.36 eV. We find the work function from KE max hf W0 ; W0 hf KEmax 4.36eV 1.40eV W0 2.96 eV.