Nuclear Chemistry Review Questions
advertisement

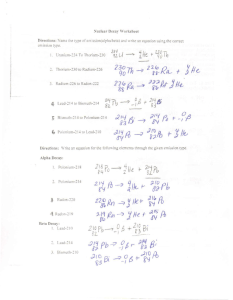
ANSWERS Nuclear Chemistry Review Questions 1. Compare the mass, charge, and penetration power of alpha, beta and gamma radiation. Alpha Beta Gamma Mass 4 0 0 Charge + 0 Penetration Lowest Middle Highest 2. In 5.49 seconds, 1.20g of argon-35 decays to leave only 0.15g. What is the half-life? 1.20g 0.60g 0.30g 0.15g , therefore it took 3 half-life periods. 5.493 = 1.83 sec 3. How many days does it take 16.0g of gold-198 to decay to 1.0g? 16.0g 8. 0g 4.0g 2.0g 1.0g , therefore 4 half-life periods From Ref Table N, 1 half-life = 2.69d 2.69d * 4 = 10.76 days 4. Why does an atom undergo radioactive decay? Because it has an unstable nucleus 5. Compare and contrast fission and fusion. Why is fission used for electrical and fusion is not? Fission=Division, the splitting of large unstable atoms(nucleus) Fusion=joining together, small isotopes of H, creates way more energy 6. How is nuclear chemistry applied to medicine? What type of radioisotope must be used when it is injected into a person? Radioisotopes are used to treat and diagnose illnesses If injected they must have short half-lives and quickly removed from the body 7. Write out the nuclear decay of radium-226. 226 4 222 88 Ra 2 He 86 Rn 8. What is different about nuclear reactions when compared to a chemical reaction? Nuclear reactions transform atoms into other atoms, chemical reactions involved the rearrangement of electrons 9. At what point do all isotopes of an element become radioactive? Up to and including atomic # 83 ANSWERS 10. Think about using nuclear power. What are three advantages and disadvantages of using this as a power source? Advantages Disadvantages -cheap power -storage of wastes -no greenhouse gases -possible radiation leaks -can supply electricity efficiently -terrorist targets 11. What is the difference between artificial and natural transmutation? How can you identify from an equation? Artificial transmutation is induced by another particle colliding with the nucleus, it can be identified by having two different reactant particles. (see b in #12) Natural transmutation is the spontaneous decay of one atom into another atom 12. Complete the following: He 11 H42 He ____ 10 e b. 3 2 1 0 c. 14 6 d. 226 88 a. 139 1 n 235 92 U 56 Ba 30 n _____ C 01 e _____ 14 7 94 36 Kr N Ra 222 86 Rn ______ 4 2 He 13. List two benefits of radioactivity for humans. You cannot use any medicinal application. Industrial measurement and food irradiation Use the paragraph below to answer questions 14-17. The first atom of element 112 was created and confirmed by a research team in 1996. The element was created by bombarding lead foil with highly ionized zinc atoms. The single atom of the new element decayed after 280 microseconds, losing an alpha particle to form element 110. 14. What is the symbol for element 112? Uub 15. Write the chemical symbol and name of an element whose chemical properties are likely to be similar to those of element 112? Explain your answer. Any element in Group 12, so Zn, Cd, or Hg 16. Show how a combination of the atomic numbers of zinc and lead could account for the formation of a nucleus of the new element described in the passage. 30Zn and 82Pb = 112Uub 17. Write the equation to show the radioactive decay of 223 227 4 112 Uub 2 He + 110 Kr 227 112 Uub as described in the passage.