Chemistry 1 Unit 3, Nuclear Chemistry Guiding Questions: What are
advertisement

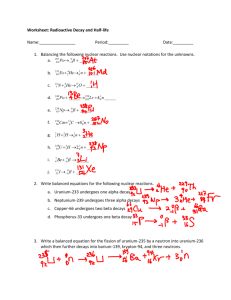
Chemistry 1 Unit 3, Nuclear Chemistry Guiding Questions: 1. What are the characteristics of alpha, beta, and gamma radiation? 2. How do we describe radioactive decay in terms of balanced nuclear equations? 3. What is the difference between fission and fusion reactions? Key Vocabulary Terms: Radioactivity Gamma Ray Fission Critical mass Alpha Particle X Ray Fusion Beta Particle Radioisotope Half-life Questions for Review 1. What is the difference between a chemical reaction and a nuclear reaction? 2. Who are the scientists associated with nuclear chemistry and what did they do? a. Wilhelm Roentgen b. Henri Becquerel c. Marie and Pierre Curie 3. What is an isotope? What is a radioisotope? 4. Name Symbol Charge Mass Energy Penetrating Power Shielding 5. Particle Proton Neutron Electron Symbol Charge Relative Mass Location 6. What are some distinguishing factors that differ between the nucleus and the electron cloud? 7. Describe the shorthand way to represent isotopes and show how to calculate protons, neutrons, and electrons. 8. What is radioactivity? 9. How can we tell if an isotope is going to be stable? 10. Which elements are always unstable? 11. What happens as a result of alpha decay? 12. Balance the following alpha decay problems: 210 84 a. Po → ______ + ______ b. 226 88Ra c. 222 86Rn → ______ + ______ → ______ + ______ 13. What happens as a result of beta decay? 14. Balance the following beta decay problems: a. 14 6C b. 97 40Zr c. 137 55Cs → _____ + ______ → ______ + ______ → ______ + ______ 15. What happens as a result of gamma decay? 16. Balance the following gamma decay problem: a. 40 19K + o -1e → _____ + o oγ 17. What is transmutation and how do we use it? 18. What is half-life and what do we use it for? 19. If you have 10.0 grams of strontium – 90, which has a half life of 29 years. How much will be remaining after 145 number of years? Number of ½ lives Time Amount Remaining 20. What is the equation for calculating half-life? 21. If gallium – 68 has a half-life of 68.3 minutes, how much of a 160.0 mg sample is left after 1 half life? ________ 2 half lives? __________ 3 half lives? __________ 22. Cobalt – 60, with a half-life of 5 years, is used in cancer radiation treatments. If a hospital purchases a supply of 30.0 g, how much would be left after 15 years? Number of ½ lives Time Amount Remaining 23. Iron-59 is used in medicine to diagnose blood circulation disorders. The half-life of iron-59 is 44.5 days. How much of a 2.000 mg sample will remain after 133.5 days? Number of ½ lives Time Amount Remaining 24. The half-life of polonium-218 is 3.0 minutes. If you start with 20.0 g, how long will it take before only 1.25 g remains? Number of ½ lives Time Amount Remaining 25. A sample initially contains 150.0 mg of radon-222. After 11.4 days, the sample contains 18.75 mg of radon-222. Calculate the half-life. Number of ½ lives Time Amount Remaining 26. What is nuclear disintegration? 27. What is nuclear fission and what is it used for? 28. What is nuclear fusion and what is it used for? 29. What is the biggest drawback of using nuclear fission and how do we deal with it?