KEY, Exam 1
Dr. Ruth Ballard
BIO 2
Page 1 Fall 2008
EXAM 1 KEY
(100 points)
NAME__________________________________ LAB SECTION # _______
b. a.
1. (12 points)
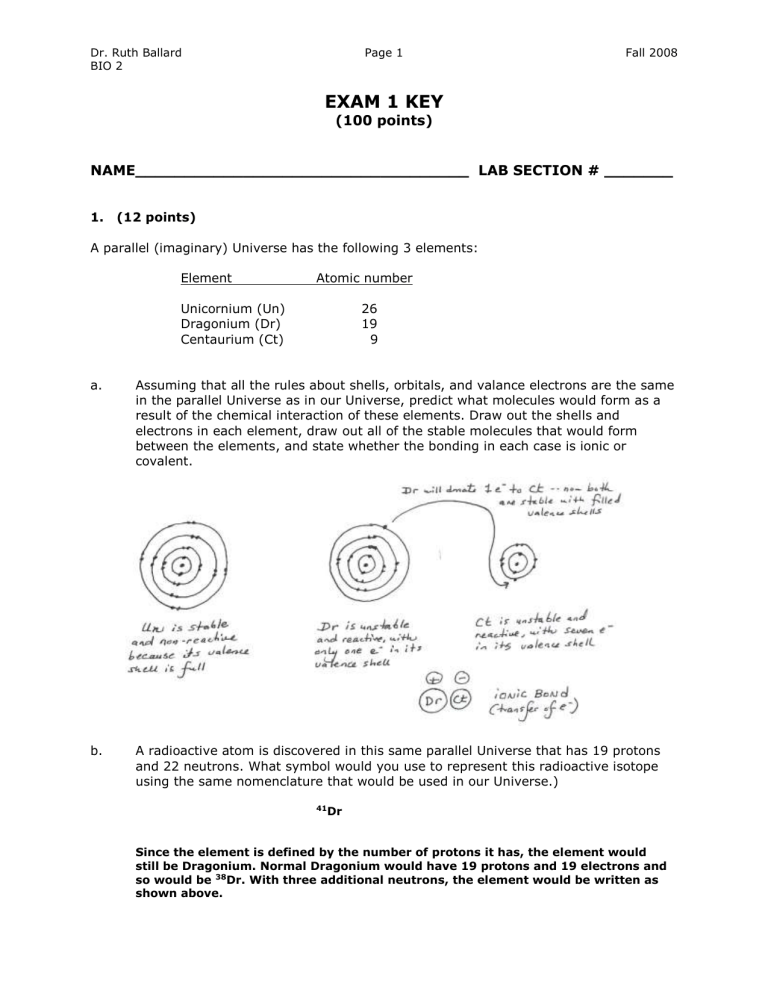
A parallel (imaginary) Universe has the following 3 elements:
Element Atomic number
Unicornium (Un)
Dragonium (Dr)
Centaurium (Ct)
26
19
9
Assuming that all the rules about shells, orbitals, and valance electrons are the same in the parallel Universe as in our Universe, predict what molecules would form as a result of the chemical interaction of these elements. Draw out the shells and electrons in each element, draw out all of the stable molecules that would form between the elements, and state whether the bonding in each case is ionic or covalent.
A radioactive atom is discovered in this same parallel Universe that has 19 protons and 22 neutrons. What symbol would you use to represent this radioactive isotope using the same nomenclature that would be used in our Universe.)
41 Dr
Since the element is defined by the number of protons it has, the element would still be Dragonium. Normal Dragonium would have 19 protons and 19 electrons and so would be 38 Dr. With three additional neutrons, the element would be written as shown above.
Dr. Ruth Ballard
BIO 2
Page 2 Fall 2008 b.
2. (8 points)
A solution has a pH of 4.9. What is the concentration of OH- ions in the solution? Show your entire calculation and logic.
3. (8 points) a. Draw out the experiment that Spallenzani performed to test the theory of abiogenesis.
What did critics (at the time) say was wrong with his experiment?
Critics argued that by capping the flask, Spallenzani had suffocated the abiogenic microbes and that is why they did not grow. Some also thought that Spallenzani might have “killed the life force” by boiling the broth.
4. (9 points)
Fill in the blanks.
The only elements that formed during the Big Bang were hydrogen, helium, lithium, and
beryllium. Heavier elements, like carbon, phosphorous, and silicon were created later, by a process called nuclear fusion in the bellies (cores) of stars. If a star is massive enough to fuse elements all the way to the element iron, the core will collapse and become a neutron
star or black hole. As a result of the collapse of the core, the outer portions of the star are blown away from the core in a massive explosion called a supernova. Life could not have evolved unless the swirling gas cloud from which the solar system formed contained the
Dr. Ruth Ballard
BIO 2
Page 3 Fall 2008 heavier elements created in this type of explosion. The four most common elements (by weight) in the human body are oxygen, carbon, hydrogen and nitrogen.
5. (4 pts)
List the four major classes of life’s large biomolecules.
Nucleic acids, Carbohydrates, Proteins, and Lipids
6. (4 pts)
What evidence from isomers of amino acids and sugars suggests that all life on Earth is related (evolved from a common ancestor)?
All life forms on Earth use right-handed (D) sugars and left-handed (L) amino acids. Since the choice of which enantomer to use is essentially random (there’s no advantage of choosing one over the other), the fact that all life forms use the same ones indicates that all life evolved from a common ancestor. Once the “choice” was made, all life forms that evolved from the original ancestor had to keep using the same enantomers, since evolution can only build on what came before – it can’t start over from scratch.
7. (10 points)
Circle all the statements below that are NOT true. a. Water has a low specific heat. h. e. f. g. d. b. c.
Water expands when it freezes.
The oxygen and hydrogen atoms in a water molecule are joined together by hydrogen bonds.
Hydrogen bonds are much weaker than covalent bonds.
Water is a non-polar molecule.
The atomic mass of water can be approximated as 16.
Water is less dense when it is solid than when it is liquid.
There is more water in the Northern Hemisphere of Earth than in the
Southern Hemisphere. i. When sugars are joined together to form a carbohydrate, water is produced.
8. (5 points)
As a member of the Challenger disaster inquiry panel, what simple experiment did physicist
Richard Feynman perform to show that O-rings shrink at cold temperatures?
During the inquiry, he asked for a glass of ice water. Then he measured the O-ring before putting it in the ice water, waited a few minutes, and then measured it again. He demonstrated that the O-ring did, in fact, shrink at low temperatures.
Dr. Ruth Ballard
BIO 2
Page 4 Fall 2008
9. (4 points)
What happened approximately 300,000 years after the Big Bang?
The Universe got cool enough for the first atoms to form (protons, neutrons, and electrons found each other to form mostly hydrogen, some helium, and a little bit of lithium and berrilium).
10. (8 pts)
List four characteristics of living organisms that are NOT characteristics of non-living things.
Living organisms separate “self” from “other” and actively defend themselves from annihilation.
Living organisms harvest the energy from solar radiation (either directly or indirectly) to build highly complex systems.
Living organisms harbor and protect an information molecule, which they pass on to their offspring.
Living organisms (and the information molecule they host) evolve over time, becoming better adapted (or at least maintaining their level of adaptation) to a changing environment.
11. (4 pts) a. Approximately how far across (in light years) is the Milky Way galaxy? 100,000 ly b. How long does it take light (traveling at 186,000 miles per second) to cross the Milky
Way galaxy? Express your answer in years.
The speed of light is 1 light-year/1 year.
(100,000 ly) (1 yr/1 ly) = 100,000 years
12. (24 points)
True (T) or False (F)? If the statement is false, cross out the underlined word or phrase and replace it with the correct word or phrase.
incorrect
EXAMPLE: Dr. Ballard will not answer e-mails with correct grammar and spelling.
_____ The solar system is about 5 billion years old. T
_____ One of the gases that was prevalent in Earth’s early atmosphere was oxygen; F;
lots of answers possible here – e.g. nitrogen, methane, hydrogen sulfide, etc.
_____ The Big Bang occurred approximately 13.8 trillion years ago.
F; billion
Dr. Ruth Ballard
BIO 2
Page 5 Fall 2008
_____ Proteins can be stabilized by disulfide bonds. T
_____ Hydroxyl groups make molecules less soluble in water. F; more
_____ An aqueous solution with a high pH has a high concentration of H+ ions. F; OH-
_____ During an ice age, the Northern hemisphere is tilted toward the Sun at aphelion. F; perihelion
_____ Carbon likes to share three electrons with other atoms. F; four
_____ Carboxyl groups make a molecule more basic. F; amino
_____ Starch is polymer of glucose monomers. T
_____ Theories never become facts. F; sometimes
_____ Sodium chloride is a hydrophobic molecule. F; hydrophilic
_____ Valence electrons are located in the outermost shell. T
_____ Large stars go through their life cycles more quickly than small stars. T
_____ A compound contains two or more elements in fixed proportions. T








