ge132_paper_dundas - Division of Geological and Planetary
advertisement

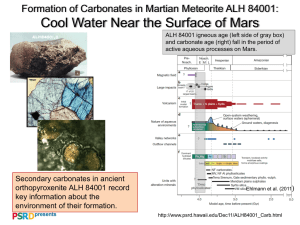
Colin Dundas Ge/Ay 132 Term Paper March 15, 2004 The Search For Carbonates on Mars The history of Mars is one of the most intriguing issues in planetary science today. Many have proposed that the planet had vast oceans in its early years similar to those of Earth today. However, these exciting theories have been difficult to prove, and face several major issues. One of the important questions relating to early oceans on Mars is that of the existence of carbonates. Carbonate deposits are expected to form in the presence of oceans and carbon dioxide, conditions which may well have existed on early Mars. Yet no large deposits of carbonates, such as might form on a seafloor, have been observed there. The amount and location of Martian carbonates is thus an important issue that has been heavily studied recently. Carbonates such as calcite (limestone) and dolomite are important parts of Earth’s rock assemblage. Net reactions like CaSiO3 + CO2 CaCO3 + SiO2 produce carbonates in water when atmospheric carbon dioxide is present, particularly on seafloors. On Earth, plate tectonics subducts carbonates and restores carbon dioxide to the atmosphere through volcanic activity, and a balance is struck between carbonate formation and removal. However, Mars lacks active plate tectonics. If Mars had an early ocean, carbonates should have formed, depleting the atmosphere. The greenhouse effect from carbon dioxide would have dwindled and the atmosphere would have become cold and thin, making the liquid-water ocean unstable and producing essentially the characteristics observed today (Morse and Marion, 1999). The carbonates would have 1 been left behind and never subducted. Hence if carbonates formed in a Martian ocean, they should still be somewhere in Mars’ crust. Carbonates are detectable by means of their infrared rotational spectrum. Several vibrational modes of carbonates produce absorption lines in the infrared which can be used to uniquely identify carbonate. The ν2 and ν3 fundamental vibrational absorptions are located at around 890 and 1540 cm-1, corresponding to out-of-plane bending and asymmetric stretches of the molecule; ν1, the symmetric stretch mode, is not observable since it doesn’t alter the dipole moment (Lane and Christensen 1998, Bandfield et al 2003). There are also overtones and combination bands at higher energies, but they are not generally used for identifying carbonates. The precise location of each band depends on the specific carbonate, but the variations are minor and the spectral signature is essentially the same for all carbonates. Figure 1, from Bandfield et al 2003, shows spectra of various carbonates mixed with labradorite, as well as Martian dust. Carbonate Formulae: Siderite: MgCO3 Calcite: CaCO3 Dolomite: [CaMg(CO3)2] Magnesite: MgCO3 (Lane and Christensen 1998) Figure 1: IR spectra of carbonate-labradorite mixes compared with Mars dust. (From Bandfield et al 2003) 2 Several different efforts have been made to detect Martian carbonates. The Mars Global Surveyor spacecraft used the Thermal Emission Spectrometer (TES) instrument to take IR spectra of most of Mars’ surface, at resolution sufficient to resolve large rock units. TES took spectra over the range 6-50 μm (200-1670 cm-1), which easily includes the carbonate lines, although the carbon dioxide in the atmosphere does block some of the range. TES results indicated that most of the surface is either basaltic or andesitic in broad composition (Bandfield et al 2000, Christensen et al 2001). There was no indication of any large carbonate units exposed anywhere on the surface, either in the possible northern ocean basin or anywhere else. TES did not rule out small or very finegrained units of carbonate, nor did it examine the subsurface composition. It did provide strong evidence that there are no large geological units of carbonate on the surface, which may undermine the ocean hypothesis. However, the present surface of the northern hemisphere is relatively young and the ocean floor could have been resurfaced, masking old carbonates. 3 Figure 2: TES spectrum of the Martian surface. There is no indication of any significant amount of carbonates, as the overall spectrum is essentially volcanic. (From Christensen et al 2001) Smaller amounts of carbonates have been observed by other means. Spectra from the Infrared Space Observatory have indicated small amounts of carbonate at wavenumbers that indicate that it is likely being detected by transmittance rather than emission (Lellouch et al 2000); hence this detection is likely of carbonate dust in Mars’ atmosphere. The assignment of this spectrum may be flawed, as the wavenumbers of the minima in the spectrum are at 7.2 and 11.1 μm, but calcite (for example) has minima at 7 and 11.4. The errors between assignment and lab spectrum would then be in the opposite direction for the two features, which is cause for some concern. However, there have been other indications of carbonates in small amounts. Detailed examination of TES spectra does yield indications of between 2-5% carbonate by weight in the dust on the surface (Bandfield et al 2003) and Martian meteorites analyzed in detail contain small amounts of carbonate as well. Although the amount of carbonates that this indicates is unclear, since the amount of dust is not well known, even a small weight % of carbonate 4 in the Martian crust could account for a thick ancient atmosphere. However, the source of the carbonate in the dust is not clear. It is possible that original large carbonate formations have now been weathered away, leaving some debris in the current dust. Alternatively, small amounts of carbonate could have formed in many rocks which were subsequently weathered. Since only the surface composition is known (and the surface is masked by dust in many places) it is difficult at the present to reach any firmer conclusions. The search for carbonates on Mars is one of the most important and informative paths towards understanding its past. Carbonates may hold clues to ancient oceans or the history of surface water. Recent results show only small amounts of carbonate material on the Martian surface, most likely in the widespread dust. There are no large, exposed deposits of carbonate rock, which could be a marker for old lakes or seafloor. However, this observation does not conclusively rule out the ocean hypothesis; carbonates could be buried or weathered away, or they might not have formed on Mars for some unforeseen reason. Carbonates do appear to constitute a few percent of the dust which coats much of the surface, and if this is representative of the upper crust then several bars of carbon dioxide could be sequestered there. Current measurements only provide information about the surface, which can be masked by very small amounts of dust. To gain a better understanding of whether Mars has carbonates and what has happened to them, more detailed information is needed not only about the surface but about the composition of the entire crust. While this may not occur for some time, it is the next step in unraveling this portion of the history of Mars. 5 References Bandfield JL, Hamilton VE, Christensen PR. 2000. A Global View of Martian Surface Composition from MGS-TES. Science 287: 1626-1630. Bandfield JL. 2002. Global Mineral Distributions on Mars. Journal of Geophysical Research—Planets 107 (E6): art. 5042. Bandfield JL, Glotch TD, Christensen PR. 2003. Spectroscopic Identification of Carbonate Minerals in the Martian Dust. Science 301: 1084-1087. Christensen PR et al. 2001. Mars Global Surveyor Thermal Emission Spectrometer Experiment: Investigation Description and Surface Science Results. Journal of Geophysical Research—Planets 106 (E10): 23823-23871. Lane MD, Christensen PR. 1998. Thermal Infrared Emission Spectroscopy of Salt Minerals Predicted for Mars. Icarus 135 (2): 528-536. Lellouch E, Encrenaz T, de Graauw T, Erard S, Morris P, Crovisier J, Feuchtgruber H, Girard T, Burgdorf M. 2000. The 2.4-45 μm Spectrum of Mars Observed with the Infrared Space Observatory. Planetary and Space Science 48 (12-14): 1393-1405. Morse JW, Marion GM. 1999. The Role of Carbonates in the Evolution of Early Martian Oceans. American Journal of Science 299 (7-9): 738-761. 6