File
advertisement
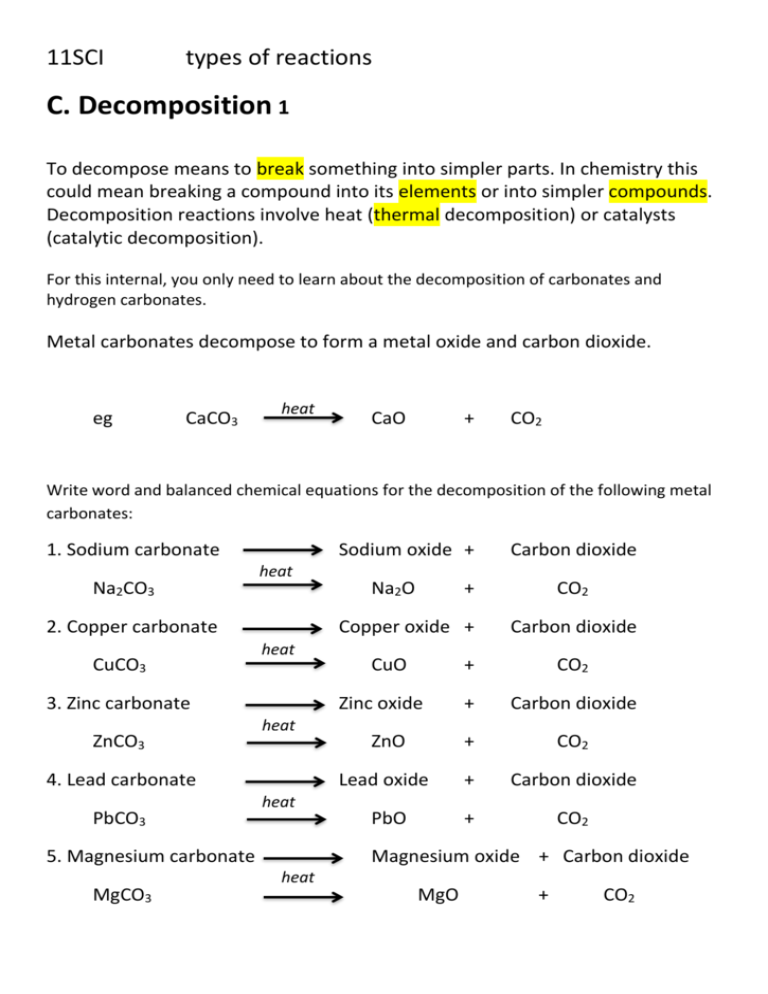
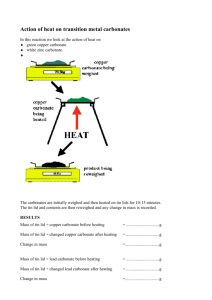
11SCI types of reactions C. Decomposition 1 To decompose means to break something into simpler parts. In chemistry this could mean breaking a compound into its elements or into simpler compounds. Decomposition reactions involve heat (thermal decomposition) or catalysts (catalytic decomposition). For this internal, you only need to learn about the decomposition of carbonates and hydrogen carbonates. Metal carbonates decompose to form a metal oxide and carbon dioxide. eg CaCO3 heat CaO + CO2 Write word and balanced chemical equations for the decomposition of the following metal carbonates: 1. Sodium carbonate Na2CO3 Sodium oxide + heat 2. Copper carbonate CuCO3 heat heat heat 5. Magnesium carbonate MgCO3 CuO CO2 Carbon dioxide + CO2 + Carbon dioxide ZnO + CO2 Lead oxide + Carbon dioxide PbO + CO2 Zinc oxide 4. Lead carbonate PbCO3 + Copper oxide + 3. Zinc carbonate ZnCO3 Na2O Carbon dioxide Magnesium oxide + Carbon dioxide heat MgO + CO2