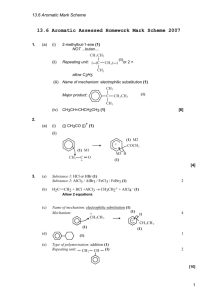
AROMATIC CHEMISTRY

AROMATIC CHEMISTRY
The term ‘aromatic’ was first used in the nineteenth century to describe a group of compounds which have pleasant aromas. These compounds, which include benzene, are very different to aliphatic compounds. The term is still used, since it is useful to classify aromatic and aliphatic compounds separately, but the word now has a much more fundamental meaning related to the
-bonding in a molecule.
BENZENE
Benzene has the molecular formula C
6
H
6
and therefore contains four double bond equivalents. However, it does not readily undergo electrophilic addition reactions, like alkenes do, and was thought at first not to contain double bonds. One of the earliest structures for benzene was suggested by Ladenburg; another suggestion came from
Dewar.
H
H
H
H
H
Ladenburg
H
Dewar
In 1865, Kekule suggested that a rapid equilibrium existed between two equivalent forms of benzene. This, he thought, would average out the double and single bonds and would explain the reluctance of benzene to undergo electrophilic addition reactions.
Other evidence about the structure of benzene:
X-ray Diffraction
C=C has a bond length of 0.134nm
C-C has a bond length of 0.154nm
X-ray diffraction analysis of benzene shows that:
the six carbon-carbon bonds are of equal length and are intermediate between double and single bonds, with a bond length of 0.139nm
the molecule is planar: all twelve atoms lie in the same plane
Enthalpy of Hydrogenation
If cyclohexene is reduced by hydrogen to cyclohexane, the observed enthalpy change is -120kJ.mol
-1
+ H
2
H = -120 kJ.mol -1
TOPIC 13.7: AROMATIC CHEMISTRY 1
If cyclohexa-1,3-diene is reduced by hydrogen to cyclohexane, the observed enthalpy change is -234kJ.mol
-1
+ 2H
2
H = -234 kJ.mol
-1
This enthalpy change, which involves the reduction of two double bonds, is as expected, approximately twice the enthalpy change observed for the reduction of one double bond.
Therefore, if the hypothetical molecule, cyclohexa-1,3,5-triene were to be reduced to cyclohexane, the expected enthalpy change would be approximately –350 kJ.mol
-1 .
+ 3H
2
H ~ -350 kJ.mol -1
However, when benzene is reduced to cyclohexane, the observed enthalpy change is much less than -350 kJ.mol
-1 , showing that benzene is much more stable than
‘cyclohexa-1,3,5-triene’, which contains 3 isolated double bonds.
+ 3H
2
H = -208 kJ.mol -1
This increased stability arises because of the delocalisation energy of benzene.
Energy
~ -145 kJ.mol
-1 delocalisation energy
~ -350 kJ.mol
-1
-208 kJ.mol
-1
TOPIC 13.7: AROMATIC CHEMISTRY 2
The Structure of Benzene
All six carbon atoms are sp 2 hybridised, and therefore have trigonal planar geometry, with a bond angle of 120 o . This gives a regular hexagon of C-C
-bonds (all bond lengths the same), with all twelve atoms (6xC 6xH) in the same plane.
Each carbon atom has, in addition, a half-filled 2p-orbital, which is perpendicular to the plane of the ring.
The six 2p-orbitals overlap to form a delocalised, cyclic
-orbital, which extends over the whole ring. The orbital has two lobes, one above the plane of the ring and one below. This delocalisation has a marked stabilising effect: the reduction in energy which it brings about is called the delocalisation energy .
For a compound to be aromatic and have delocalisation energy, the
-orbital must:
contain 4n + 2
electrons (Huckel’s Rule), where n is an integer.
be cyclic
be planar
Electrophilic Substitution
The benzene ring is a centre of high electron density and will attack electron-deficient species: electrophiles. However, if benzene were to undergo electrophilic addition, like alkenes, the stability associated with the delocalisation energy would be lost.
Instead, it undergoes electrophilic substitution and retains the delocalisation energy.
As is usual with an sp 2 hybridised carbon atom, the first step in the mechanism is addition (delocalisation energy is lost), but this is followed, in the second step, by the elimination of a proton, which restores the delocalised
-system.
E +
H
E
E
+
+ H +
The overall result is substitution.
TOPIC 13.7: AROMATIC CHEMISTRY 3
Examples of Electrophilic Substitution
1. Nitration
Nitration introduces a nitro group (-NO
2
) into the benzene ring. For example, benzene is nitrated when treated with a mixture of concentrated nitric acid and concentrated sulphuric acid . The reaction is highly exothermic. The reaction temperature is allowed to rise to about 50 o C , and the mixture is then cooled to retain this temperature.
NO
2
+ HNO
3
+ H
2
O
nitrobenzene
The electrophile is the nitronium ion, NO
2
+ , which is generated by the reaction of concentrated nitric acid and concentrated sulphuric acid.
HNO
3
+ 2H
2
SO
4
2HSO
4
+ H
3
O
Initially, nitric acid is protonated by the sulphuric acid:
+ + NO
2
+
HNO
3
+ H
2
SO
4
HSO
4
+ H
2
NO
3
+ base acid
H
2
NO
3
+ then breaks down to give water and a nitronium ion:
H
2
NO
3
+ H
2
O + NO
2
+
Finally, the water molecule is protonated by a second molecule
of sulphuric acid.
H
O
:O
Mechanism:
+
NO
2
+
H
NO
2
NO
2
+ H +
Uses of nitro compounds
Nitration is used to manufacture explosives such as T.N.T. (2,4,6trinitromethylbenzene)
Nitro compounds can be reduced to aromatic primary amines by reduction with
Sn/conc. HCl or Ni/H
2
. Aromatic primary amines are used widely in the manufacture of dyestuffs, because amino groups are auxochromes.
TOPIC 13.7: AROMATIC CHEMISTRY 4
2. Acylation (Friedel-Crafts reaction)
Acylation involves the introduction of an acyl group (RCO) into the benzene ring and therefore involves the formation of a new carbon-carbon bond. The electrophile required is RCO + .
The reaction is carried out at room temperature by treating benzene with an acyl chloride in the presence of AlCl
3
. The product is a ketone.
COR
AlCl
3
+ RCOCl + HCl
Mechanism:
The acyl chloride, RCOCl, forms a coordinate bond with the AlCl
3
. This has the effect of increasing the polarisation of the C-Cl bond, forming an ion pair.
RCOCl + AlCl
3
RCO + + AlCl
4
-
acylium ion
The acylium ion is sufficiently electrophilic to be attacked by the benzene
-system.
+
COR
+
H
COR
Finally, the hydrogen ion reacts with AlCl
4
to regenerate the catalyst.
H + + AlCl
4
HCl + AlCl
3
COR
+ H +
Friedel-Crafts acylation reactions can be carried out using carboxylic acid anhydrides in place of acyl chlorides. One of the first steps in the synthesis of anthraquinone dyes is the acylation of benzene with benzene-1,2-dicarboxylic anhydride in the presence of AlCl
3
.
TOPIC 13.7: AROMATIC CHEMISTRY 5